Korean wild chive (
Allium monanthum) is a bulbous geophyte belonging to the family Amaryllidaceae. It is native to China North-Central, Japan, Korea, Manchuria, and Primorye (
Plants of the World Online, 2023). In Korea, the plant originally grows naturally, but it is cultivated as a vegetable crop by farmers and used for side dishes and cooking. In May 2020, we surveyed disease occurrence on vegetables grown in Seosan area, Korea. During the disease survey, white rot symptoms were observed in Korean wild chive plants growing in fields. The symptoms occurred mainly in the seed bulb-producing fields of the crop. The above ground parts of the diseased plants displayed premature yellowing and dying of older leaves and stunting of the plants (
Fig. 1A). The bulbs and roots of the diseased plants turned black and rotted, and white mycelia and crusted black sclerotia were formed on the bulbs (
Fig. 1B-
D). The survey of the white rot outbreak was conducted at three sites in a field. As the result of the survey of 100 plants per site, the disease occurred in a range of 1-60% in four of the eight fields surveyed.
Fig. 1.
White rot symptoms of Korean wild chive plants. (A‒ C) Symptoms of white rot observed in the fields. (D) Symptoms of the disease on the bulbs. (E‒ G) Symptoms induced 60 days after artificial inoculation with Stromatinia cepivora isolate. (H, I) Control plants.

Diseased bulbs of Korean wild chive were collected from the fields during the surveys. Fungi were isolated from the diseased bulbs collected. The 3-5 mm-long lesion pieces cut from the diseased bulbs were plated on 2% water agar after surface-sterilizing with 1% sodium hypochlorite solution for one min. The fungal mycelia growing from the lesion pieces were transferred to potato dextrose agar (PDA) slants after incubating the plates at 20°C for 2-3 days. Three isolates (AMSC-2001, AMSC-2007, and AMSC-2009) of
Sclerotium sp. were obtained from the lesion pieces and cultured on PDA at 20°C for 15 days. The colonies of the isolates on PDA consisted of white to whitish-gray mycelia and many small sclerotia (
Fig. 2A). Sclerotia were black, spherical (
Fig. 2B), and measured 300-600 μm in diameter. Conidia and ascospores were not formed on the medium. The morphological characteristics of the isolates were similar to those of
Sclerotium cepivorum described in a previous study (
Mordue, 1976) (
Table 1).
S. cepivorum was designated as a synonym of
Stromatinia cepivora (
Whetzel, 1945). The current name of
S. cepivorum is used as
S. cepivora because typification of
S. cepivorum was not indicated (
Index Fungorum, 2023).
Fig. 2.
Morphological features of Stromatinia cepivora isolated from Korean wild chive. (A) A colony of the fungus grown on potato dextrose agar (PDA) at 20°C for 15 days. (B) Sclerotia produced on PDA.
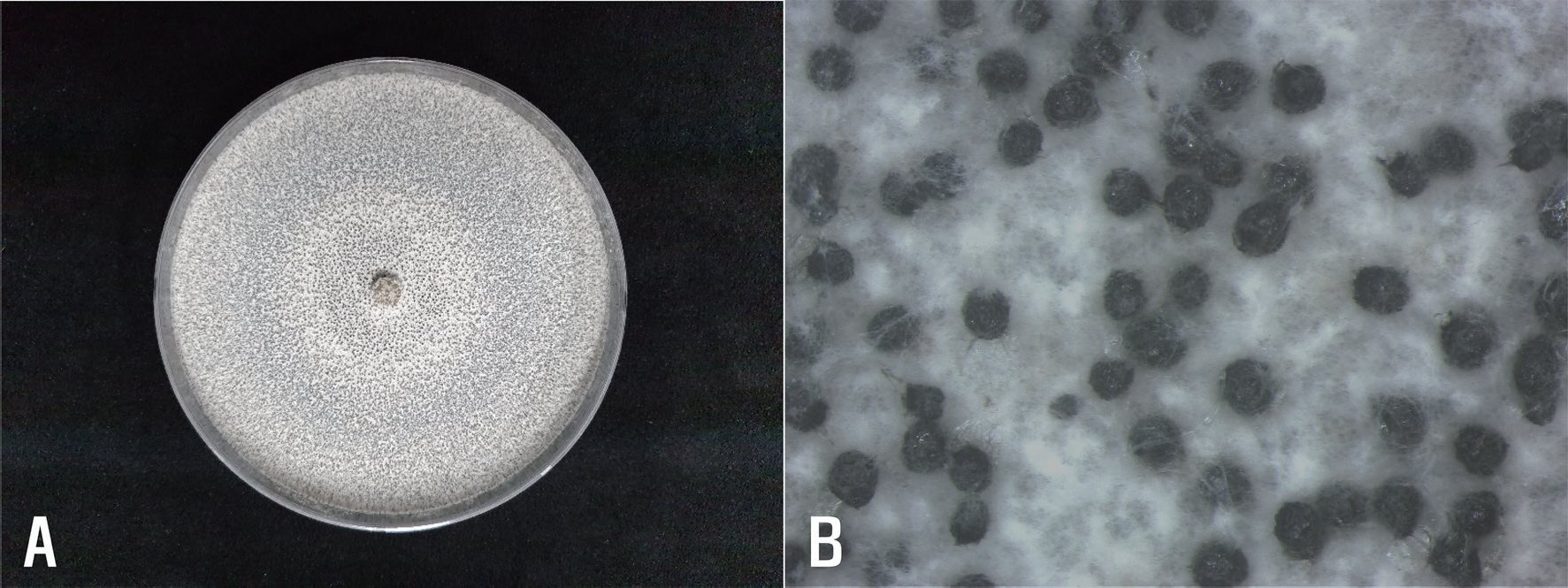
Table 1.
Morphological characteristics of Sclerotium sp. isolates from Korean wild chive and Sclerotium cepivorum described in a previous report
|
Structure |
Present isolates |
S. cepivorum (Mordue, 1976) |
|
Colony on potato dextrose agar |
White to whitish-gray |
White or faintly brownish-grey |
|
Primary hyphae |
Septate, 7-13 μm wide |
Septate, 9-14 μm wide |
|
Sclerotia |
Black, spherical, 300-600 μm in diameter, with smooth or slightly pitted surface |
Black, near spherical, 200-500 μm in diameter, with smooth or shallowly pitted surface |
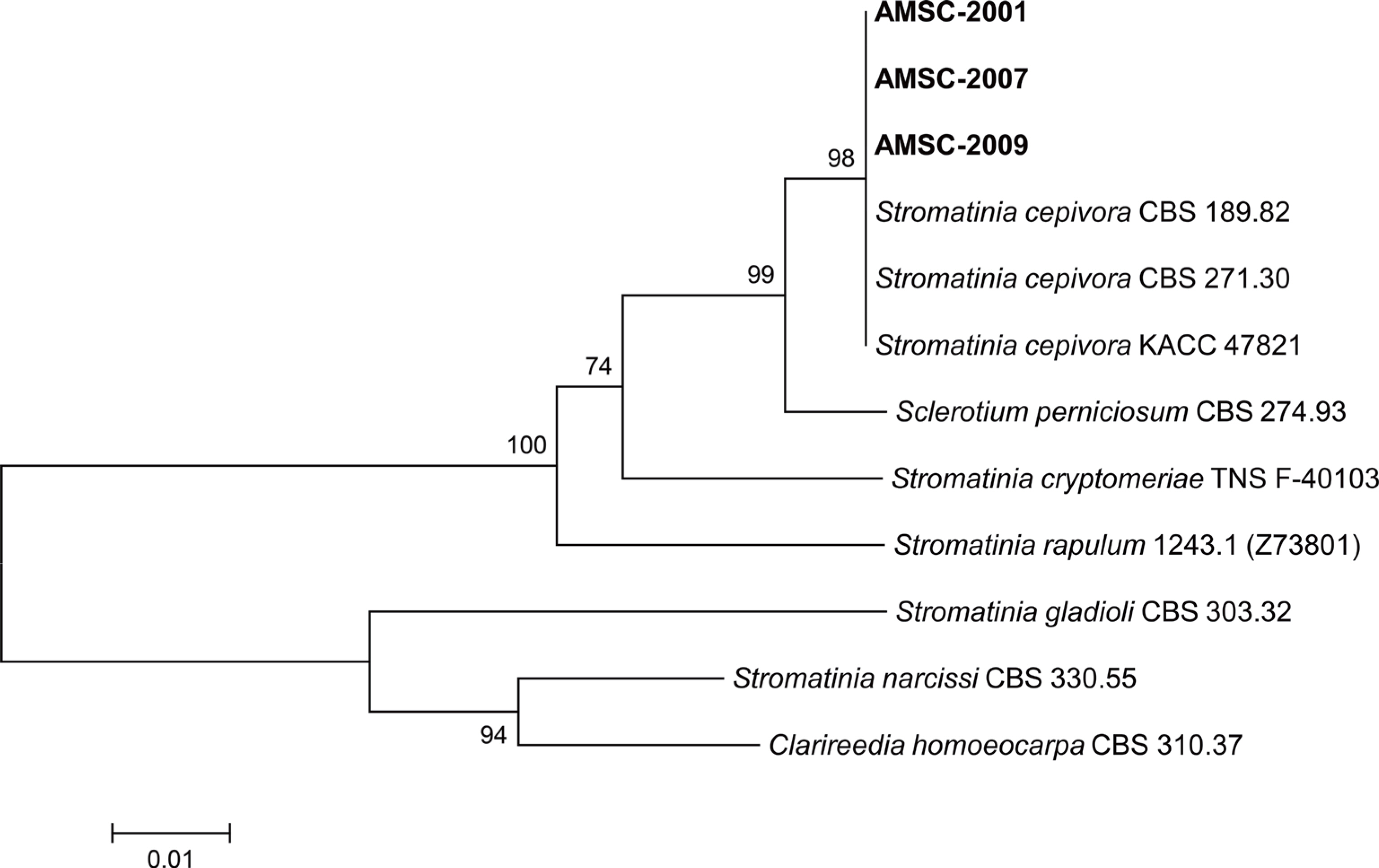
To verify the identification result based on the morphological characteristics, phylogenetic analysis was conducted. DNA of the isolates was extracted using the previous protocol (
Dong et al., 2022). Internal transcribed spacer regions 1 & 2 and intervening 5.8S nrDNA (ITS) was investigated using primers ITS1 and ITS4 (
White et al., 1990). Polymerase chain reaction (PCR) amplification was done using DNA Free-Multiplex Master Mix (Cellsafe, Yongin, Korea), following the manufacturer's instructions. The PCR products were purified using Universal DNA Purification Kit (Tiangen, Beijing, China), following the manufacturer's instructions. The purified PCR products were sequenced at Bionics (Seoul, Korea). The obtained sequences of the isolates were aligned using MUSCLE (
Edgar, 2004). A phylogenetic tree was constructed using the neighbor-joining method with a maximum composite likelihood model including 1,000 bootstrap replicates using MEGA version 7 software (
Kumar et al., 2016).
Clarireedia homoeocarpa CBS 310.37 was utilized as an outgroup taxon. Sequence data of
Stromatinia spp. and
Sclerotium perniciosum were obtained from the National Center for Biotechnology Information (NCBI) GenBank database. The phylogenetic tree represented that the isolates were grouped with
S. cepivora strains CBS 189.82, CBS 271.30, and KACC 47821 (
Fig. 3). Accordingly, all isolates from Korean wild chive were identified as
S. cepivora based on the phylogenetic analysis. The nucleotide sequences of the three isolates were deposited in NCBI GenBank with accession numbers of OQ552828- OQ552830.
Fig. 3.
Phylogenetic tree based on internal transcribed spacer regions 1 & 2 and intervening 5.8S nrDNA region of Stromatinia cepivora isolates (AMSC-2001, AMSC-2007, and AMSC-2009) from Korean wild chive and other species in the genera Stromatinia and Sclerotium. Se-quences of the reference species were obtained from the NCBI GenBank database. The tree was generated using the neighbor-joining method with a maximum composite likelihood model. The bootstrap support values are given at the nodes. The scale bar represents the number of nucleotide substitutions per site.
The three isolates of
S. cepivora were tested for pathogenicity on Korean wild chive plants by artificial inoculation. Each isolate was cultured on cornmeal-sand medium (23 g cornmeal+210 g sand+60 ml distilled water) in 500 ml-flasks at 20°C for 60 days to prepare inoculum of the isolate. Seed bulbs of Korean wild chive were surface-sterilized with 1% sodium hypochlorite solution for 3 min and washed three times with sterile distilled water. The seed bulbs were placed between wet paper towels in a plastic box and incubated at 20°C for 5 days. After the incubation, 3 germinated seed bulbs were sown in the soil at a depth of 5-6 cm in a circular plastic pot (height, 15 cm; upper diameter, 17 cm; lower diameter, 10 cm). A 50 g of each inoculum was placed on the germinated seed bulbs, and soil was covered 2-3 cm thick on the inoculum. The same quantity of cornmeal-sand medium was used for the control. The inoculated seed bulb pots were placed under alternating cycles of 12 hr LED (light emitting diode) light and 12 hr darkness in a cultivation room at 18‒22°C. The inoculation test was performed in triplicate. The result of pathogenicity tests was investigated 60 days after inoculation. All tested isolates of
S. cepivora induced white rot symptoms in the inoculated seed bulbs (
Fig. 1E-
G), but no symptoms were observed in the non-inoculated seed bulbs (
Fig. 1H,
I). The lesions induced by the inoculation test were similar to those observed in the investigated fields. The inoculated isolates were re-isolated from the lesions.
It is known that
S. cepivora causes white rot in
Allium spp. (
Crowe, 2008;
Mordue, 1976). In Korea, white rot has been reported to occur in garlic, onion, Welsh onion, and shallot (
Cho and Kim, 1996). However, there has been no report of the disease incidence on Korean wild chive. This is the first report of
S. cepivora causing white rot in Korean wild chive.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print