Meliosma myriantha Siebold & Zucc. (Sabiaceae), a decidu-ous broad-leaved tree is native to the temperate regions of East Asia, including China, Japan, and Korea, and is distributed mainly in mountain and coastal forests. In Korea, this tree grows primarily in forests along the southwestern coast but has recently been designated as a tree species that is vul-nerable to climate change (
Kwon et al., 2012). Interestingly,
M. myriantha was noted for its antioxidative and ╬▒-amylase in-hibitory activities and anti-cancer effects (
Oh and Koh, 2009;
Yoo, 2020).
Rust disease on
M. myriantha has occurred only in East Asia and is attributed to three different rust fungi, namely,
Neophysopella meliosmae (Kusano) Jing X. Ji & Kakish.,
N. meliosmae-myrianthae (Henn. & Shirai) Jing X. Ji & Kakish., and
N. vitis (P. Syd.) Jing X. Ji & Kakish. (
Ji et al., 2019;
Ono, 2000).
Neophysopella meliosmae is an autoecious species that completes its life cycle only on
M. myriantha. The latter two species are heteroecious, forming spermogonial and aecial stages on
M. myriantha, but their uredinial and telial hosts are different:
Vitis spp. for
N. meliosmae-myrianthae and
Parthenocissus tricuspidata (common name: Boston ivy) for
N. vitis (
Chatasiri and Ono, 2008;
Ono, 2000). In Korea, the rust caused by
N. vitis on Boston ivy is highly prevalent and severe, but the rust on Virginia creeper (
Parthenocissus quinquefolia) was first noticed in 2021 (
Na et al., 2022).
After rust symptoms on
M. myriantha were found at the Jeolmul Natural Recreation Forest, Jeju, Korea, in June 2010, this disease has been continuously observed in several southern regions and islands of Korea, including Seogwipo, Boseong, Gangjin, Wando, and Geoje. Disease incidence was severe, often exceeding 90% among the trees surveyed. Symptoms appeared as pale yellow to bright green spots on the upper leaf surface (
Fig. 1A), and yellow to orange-colored aecia became visible on the abaxial surface (
Fig. 1B). As the disease progressed, the aecia disappeared, and the lesions turned brown (
Fig. 1C). The present study aimed to record this rust disease on
M. myriantha in Korea and to identify the causal fungus based on morphological characteristics, DNA sequence data, and a pathogenicity test.
Fig.┬Ā1.
Rust disease caused by Neophysopella vitis on Meliosma myriantha and its pathogenicity test on Boston ivy (Parthenocissus tricuspidata) and Virginia creeper (Parthenocissus quinquefolia). (A) Rust symptoms on the upper leaf surface. (B) Aecia on the lower surface of M. myriantha. (C) Brown spots at the later disease stage on the lower leaf surface of M. myriantha. (D) Spermogonia on the upper leaf of M. myriantha. (E) Aecia on the lower leaf of M. myriantha. (F) Aeciospores. (G-I) Uredinia formed by artificial inoculation of N. vitis aeciospores harvested from Meliosma myriantha onto Boston ivy (G) and Virginia creeper (H, the upper leaf surface; I, the corresponding lower leaf surface). The arrow in (H) means the rut spots on the upper leaf surface. Scale bars=500 ┬Ąm (D, E), 10 ┬Ąm (F).

For morphological investigation, rust-infected leaves of
M. myriantha were observed under a dissecting microscope (M205C, Leica, Wetzlar, Germany), a DIC light microscope (Axio Imager 2, Carl Zeiss, Oberkochen, Germany) and a scanning electron microscope (S-4800+EDS, Hitachi, Tokyo, Japan). A total of eight voucher specimens were deposited at the Korea University Herbarium (KUS-F) in Seoul and the Kunsan National University Herbarium (KSNUH) in Gunsan, Korea (
Table 1).
Table┬Ā1.
List of the herbarium specimens of rust-infected Meliosma myriantha
|
Herbarium no. |
Host |
Date |
Location |
GenBank accession no. |
|
ITS |
LSU |
|
KUS-F24957 |
Meliosma myriantha
|
14 Jun 2010 |
Jeolmul Natural Recreation Forest, Jeju-si (33┬░26ŌĆ▓08"N, 126┬░37ŌĆ▓31"E) |
OM423802 |
OM420267 |
|
KUS-F28615 |
Meliosma myriantha
|
21 May 2015 |
Cheonji-dong, Seogwipo-si (33┬░23ŌĆ▓02"N, 126┬░36ŌĆ▓51"E) |
OM423811 |
OM420268 |
|
KSNUH0433 |
Meliosma myriantha
|
15 May 2019 |
Mt. Jeamsan, Boseong-gun (34┬░41ŌĆ▓21"N, 126┬░59ŌĆ▓19"E) |
OM423812 |
OM420271 |
|
KSNUH1853 |
Meliosma myriantha
|
3 Jun 2022 |
Jeonju Arboretum, Jeonju-si (35┬░52ŌĆ▓16"N, 127┬░03ŌĆ▓12"E) |
OQ161600 |
OQ161599 |
|
KSNUH1869 |
Meliosma myriantha
|
18 Jun 2022 |
Samgeo-dong, Geoje-si (34┬░50ŌĆ▓15"N, 128┬░39ŌĆ▓33"E) |
OQ304336 |
OQ283684 |
|
KSNUH1929 |
Meliosma myriantha
|
30 Jun 2022 |
National Bangjangsan Natural Recreation Forest, Jangseong-gun (35┬░26ŌĆ▓37"N, 126┬░45ŌĆ▓04"E) |
OQ304335 |
OQ283685 |
|
KSNUH1938 |
Meliosma myriantha
|
7 Jul 2022 |
Mt. Jogyesan, Suncheon-si (34┬░59ŌĆ▓33"N, 127┬░20ŌĆ▓30"E) |
OQ304333 |
OQ283683 |
|
KSNUH1940 |
Meliosma myriantha
|
7 Jul 2022 |
Dadohaehaesang National Park, Wando-gun (34┬░17ŌĆ▓54"N, 126┬░42ŌĆ▓22"E) |
OQ304334 |
OQ283686 |
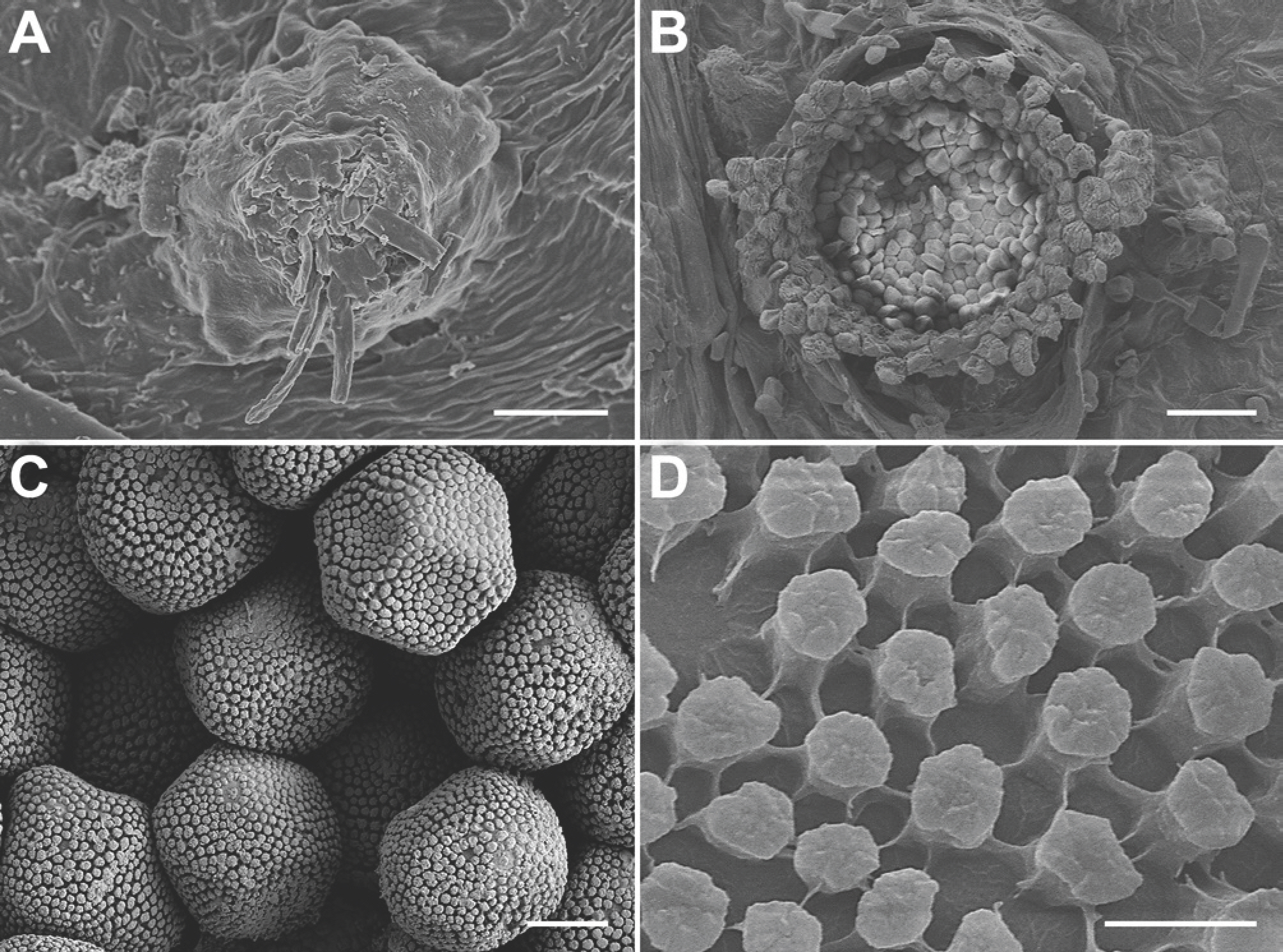
On the upper surface of
M. myriantha, spermogonia were mostly epiphyllous but rarely hypophyllous, clustered in pale yellowish-yellow lesions, brown, subepidermal, and (54.0-)58.2-82.4(-91.0) ┬Ąm (average 70.3 ┬Ąm) in diameter (
n=50) (
Figs. 1D,
2A). Aecia were hypophyllous, grouped in yellow lesions, surrounded by a well-developed peridium, and (381-)413-488(-547) ┬Ąm (average 450 ┬Ąm) in diameter (
n=50) (
Figs. 1E,
2B). Aeciospores were hyaline, mostly globose to subglobose but often angular, and (12.1-)13.7-15.7(-16.8)├Ś(16.5-)18.0-21.4(-23.5) ┬Ąm (average 14.7 ├Ś 19.7 ┬Ąm) (
n=70) (
Fig. 1F), with verrucose wall ornamentation (
Fig. 2C,
D). The morphological characteristics were consistent with those of
N. vitis (
Ono, 2000).
Fig.┬Ā2.
Neophysopella vitis on Meliosma myriantha, observed under a scanning electron microscope. (A) Spermogonium. (B) Aecium. (C) Aeciospores. (D) Wall surface of an aeciospore. Scale bars=15 ┬Ąm (A), 50 ┬Ąm (B), 5 ┬Ąm (C), 1 ┬Ąm (D).
For molecular identification, genomic DNA was extracted from aeciospores on a diseased leaf of the eight rust herbarium specimens of
M. myriantha using MagListo 5M plant Genomic DNA Extraction Kit (Bioneer, Daejeon, Korea). The internal transcribed spacer (ITS) and the large subunit (LSU) rDNA were amplified as described in (
Aime, 2006;
Beenken et al., 2012;
Na et al., 2022;
Pfunder et al., 2001). The PCR products were purified using AccuPrep PCR/Gel Purification Kit (Bioneer) and sequenced by a DNA sequencing service (Macrogen, Seoul, Korea). The assembled sequences were deposited in GenBank (
Table 1). The sequences of individual markers, including the reference sequences of
Neophysopella species retrieved from GenBank, were aligned and concatenated in MEGA X (
Kumar et al., 2018). Phylogenetic tree was inferred by the maximum-likelihood method using MEGA X, with the default settings of the program, with the Tamura-Nei model. The robustness of individual branches was esti-mated by 1,000 bootstrap replicates.
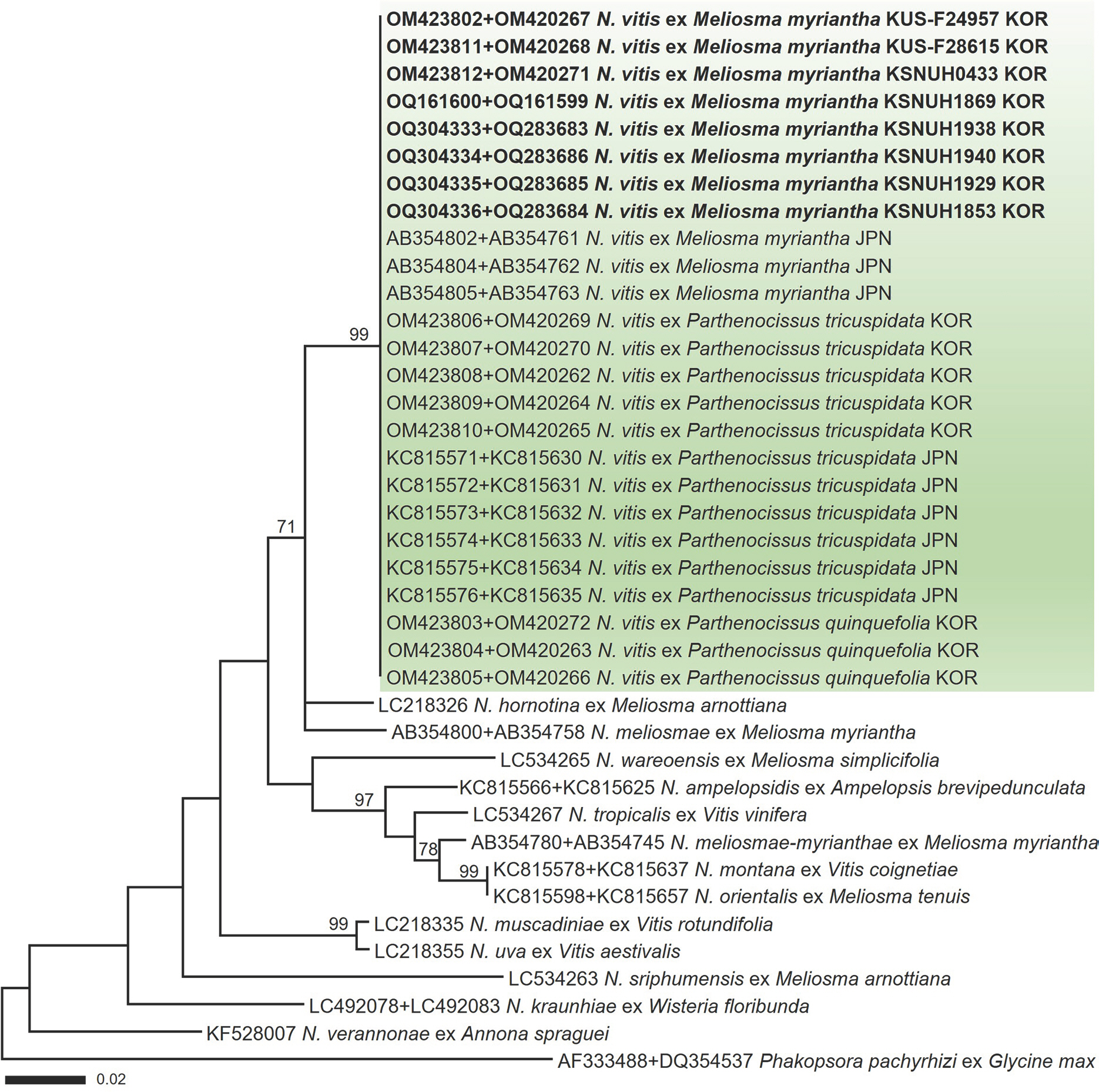
In a maximum-likelihood phylogenetic tree (
Fig. 3) of a concatenated alignment of ITS and LSU rDNA sequences, the Korean specimens formed a well-supported clade with
N. vitis sequences ex
M. myriantha, Boston ivy, and Virginia creeper, with a high bootstrap value of 99%. In the BLASTn search, the ITS and LSU sequences of the Korean specimens exhibited no nucleotide differences from those within the same phylogenetic clade.
Fig.┬Ā3.
Maximum-likelihood tree of Neophysopella species inferred from a concatenated alignment of internal transcribed spacer and large subunit rDNA sequences. The numbers above the branches represent bootstrap values over 70%. The green box represents Neophysopella vitis. The rust specimens sequenced in the present study are shown in bold.
For a pathogenicity test, mature aeciospores were harvested from about 100 aecia on naturally diseased leaves of M. myriantha, suspended in 10 ml of distilled water, and stored at room temperature (ca. 22 ┬░ C) for 1 hr. The suspension (106 aeciospores/ml) was inoculated on the lower surface of three-month-old Boston ivy (Parthenocissus tricuspidata) and Virginia creeper (Parthenocissus quinquefolia) plants. Three plants per Boston ivy and Virginia creeper were included as controls and sprayed with distilled water. Afterwards, the plants were individually covered with a plastic bag during the assay to maintain high humidity, kept in a dew chamber at 18 ┬░ C for two days in darkness, and then transferred to a plant growth chamber (22 ┬░ C, 80% humidity, a 12 hr light/12 hr dark cycle). Distilled water was sprayed daily into the plastic bags to maintain high humidity.
At ten days post-inoculation with the aeciospores of
N. vitis onto Boston ivy and Virginia creeper, yellow to orange uredinia appeared on all inoculated plants (
Fig. 1G-
I), resulting in identical symptoms to the pathogen observed previously on Boston ivy and Virginia creeper in the field. In addition, the pathogen was morphologically and molecularly identified as
N. vitis, fulfilling the Koch's postulate. Control plants remained asymptomatic.
Based on morphological investigation, sequence analysis, and pathogenicity assay,
N. vitis was identified as the causal agent of rust disease on
M. myriantha. On
M. myriantha, N. meliosmae and
N. vitis have been reported in Japan, and
N. meliosmae-myrianthae (or under its synonym
Aecidium meliosmae-myrianthi) in China, Japan, and Korea (
Farr and Rossman, 2023). This is the first report of rust disease caused by
N. vitis on
M. myriantha in Korea.
Virginia creeper is widely cultivated in many countries for ornamental purposes. Our inoculation test confirmed the pathogenicity of
N. vitis (from
M. myriantha) on Boston ivy and Virginia creeper, proving that both plants can serve as uredinial and telial hosts for
N. vitis. This was expected be-cause
M. myriantha was already known as an alternate host, in association with rust occurrence on Boston ivy, a closely re-lated species to Virginia creeper. The first global emergence of rust disease on Virginia creeper was observed in Korea in 2021 (
Na et al., 2022). Given that the geographic distribution of
M. myriantha is limited to East Asia,
N. vitis may still pose a low potential risk to Virginia creepers in other countries. This finding will help design control measures against this rust disease and conserve
M. myriantha.










