Abeysinghe, D. T., Kumara, K. A. H., Kaushalya, K. A. D., Chandrika, U. G. and Alwis, D. D. D. H. 2021. Phytochemical screening, total polyphenol, flavonoid content,
in vitro antioxidant and antibacterial activities of Sri Lankan varieties of
Murraya koenigii and
Micromelum minutum leaves.
Heliyon 7: e07449.



Adnan, M., Siddiqui, A. J., Jamal, A., Hamadou, W. S., Awadelkareem, A. M., Sachidanandan, M. et al. 2021. Evidence-based medicinal potential and possible role of
Selaginella in the prevention of modern chronic diseases: ethnopharmacological and ethnobotanical perspective.
Rec. Nat. Prod. 15: 330-355.

Arulprakasam, K. R. and Dharumadurai, D. 2021. Genome mining of biosynthetic gene clusters intended for secondary metabolites conservation in actinobacteria.
Microb. Pathog. 161: 105252.


Baek, D., Rokibuzzaman, M., Khan, A., Kim, M. C., Park, H. J., Yun, D. J. et al. 2019. Plant-growth promoting
Bacillus oryzicola YC7007 modulates stress-response gene expression and provides protection from salt stress.
Front. Plant Sci. 10: 1646.


Baillo, E. H., Kimotho, R. N., Zhang, Z. and Xu, P. 2019. Transcription factors associated with abiotic and biotic stress tolerance and their potential for crops improvement.
Genes (Basel) 10: 771.



Bratovanov, E. V., Ishida, K., Heinze, B., Pidot, S. J., Stinear, T. P., He-gemann, J. D. et al. 2020. Genome mining and heterologous expression reveal two distinct families of lasso peptides highly conserved in endofungal bacteria.
ACS Chem. Biol. 15: 1169-1176.


Cheng, C. and Hua, Z. C. 2020. Lasso peptides: heterologous production and potential medical application.
Front. Bioeng. Biotechnol. 8: 571165.



Chun, J., Oren, A., Ventosa, A., Christensen, H., Arahal, D. R., Da Cos-ta, M. S. et al. 2018. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes.
Int. J. Syst. Evol. Microbiol. 68: 461-466.


De Sousa, D. P., De Almeida Soares Hocayen, P., Andrade, L. N. and Andreatini, R. 2015. A systematic review of the anxiolytic-like effects of essential oils in animal models.
Molecules 20: 18620-18660.



de Souza Pedrosa, G. T., de Souza, E. L., de Melo, A. N. F., da Cruz Almeida, E. T., de Sousa Guedes, J. P., de Carvalho, R. J. et al. 2020. Physiological alterations involved in inactivation of autochtho-nous spoilage bacteria in orange juice caused by Citrus essential oils and mild heat.
Int. J. Food Microbiol. 334: 108837.


Etesami, H. and Beattie, G. A. 2018. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops.
Front. Microbiol. 9: 148.



Eyles, T. H., Vior, N. M., Lacret, R. and Truman, A. W. 2021. Under-standing thioamitide biosynthesis using pathway engineering and untargeted metabolomics.
Chem. Sci. 12: 7138-7150.



Funabashi, M., Funa, N. and Horinouchi, S. 2008. Phenolic lipids synthesized by type III polyketide synthase confer penicillin resistance on. Streptomyces griseus. J. Biol. Chem. 283: 13983-13991.
Gupta, S., Chaturvedi, P., Kulkarni, M. G. and Van Staden, J. 2020. A critical review on exploiting the pharmaceutical potential of plant endophytic fungi.
Biotechnol. Adv. 39: 107462.


Han, L., Yang, G., Zhou, X., Yang, D., Hu, P., Lu, Q. et al. 2013.
Bacillus thermocopriae sp. nov., isolated from a compost.
Int. J. Syst. Evol. Microbiol. 63: 3024-3029.


Hara, M. 2020. Potential use of essential oils to enhance heat tolerance in plants.
Z. Naturforsch. C J. Biosci. 75: 225-231.


Harwood, C. R., Mouillon, J. M., Pohl, S. and Arnau, J. 2018. Second-ary metabolite production and the safety of industrially important members of the
Bacillus subtilis group.
FEMS Microbiol. Rev. 42: 721-738.



Heyrman, J., Vanparys, B., Logan, N. A., Balcaen, A., Rodríguez-Díaz, M., Felske, A. et al. 2004.
Bacillus novalis sp. nov.,
Bacillus vireti sp. nov.,
Bacillus soli sp. nov.,
Bacillus bataviensis sp. nov. and
Bacillus drentensis sp. nov., from the Drentse A grasslands.
Int. J. Syst. Evol. Microbiol. 54: 47-57.


Hingston, P., Chen, J., Dhillon, B. K., Laing, C., Bertelli, C., Gannon, V. et al. 2017. Genotypes associated with
Listeria monocytogenes isolates displaying impaired or enhanced tolerances to cold, salt, acid, or desiccation stress.
Front. Microbiol. 8: 369.



Jiang, L., Jeon, D., Kim, J., Lee, C. W., Peng, Y., Seo, J. et al. 2021. Pyo-melanin-producing
Brevundimonas vitisensis sp. nov., isolated from grape (
Vitis vinifera L.).
Front. Microbiol. 12: 733612.



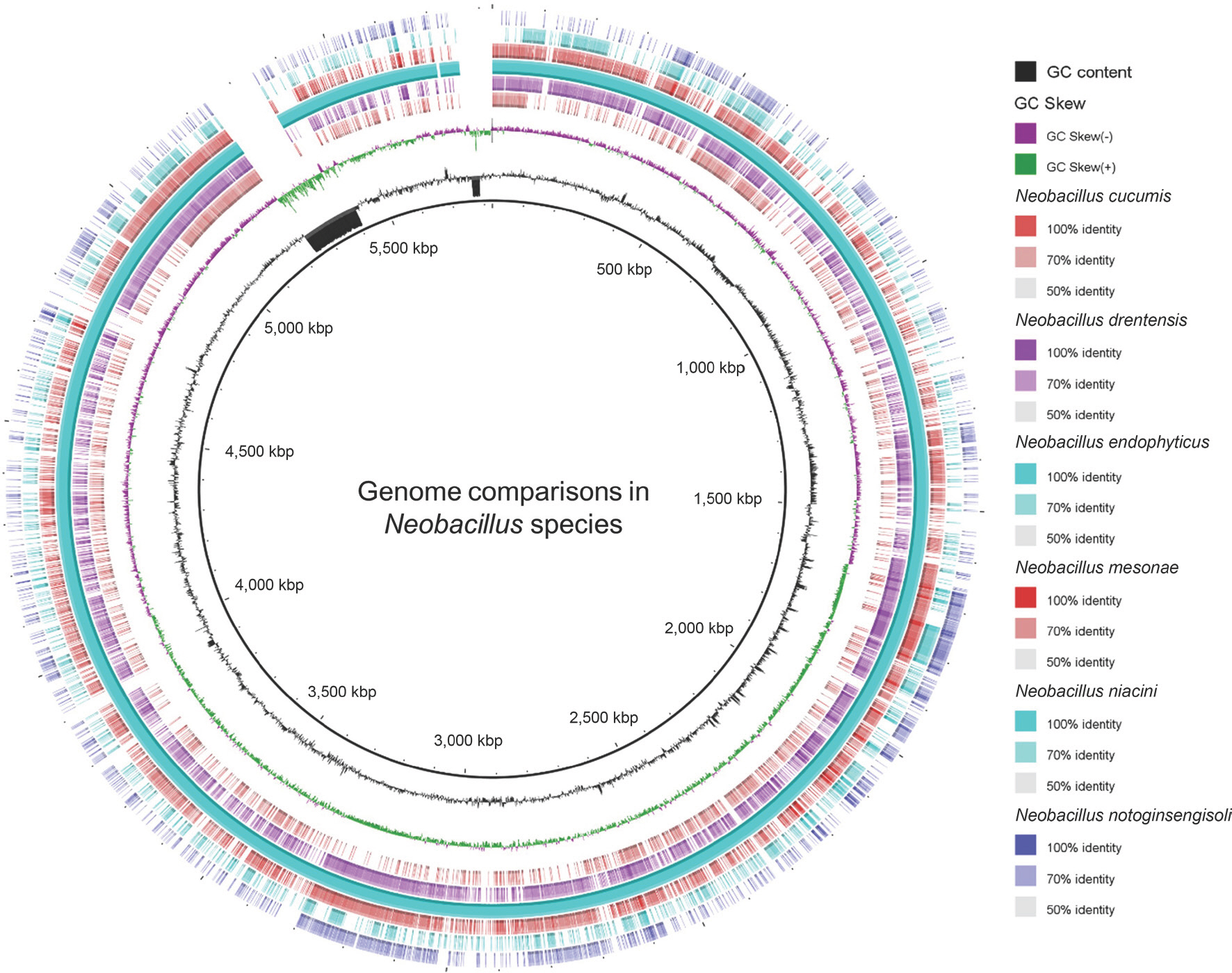
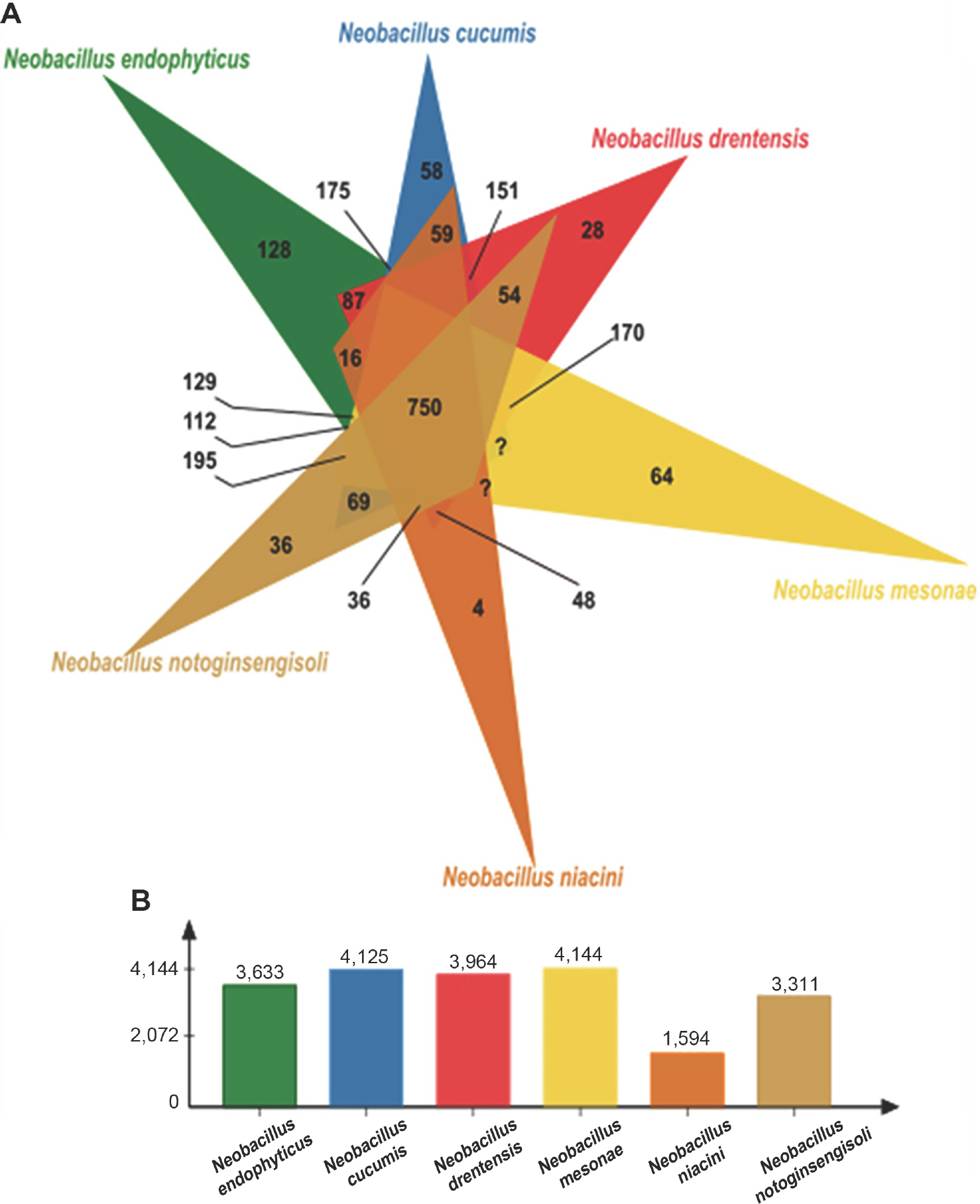
Jiang, L., Lee, M. H., Jeong, J. C., Kim, D. H., Kim, C. Y., Kim, S. W. et al. 2019.
Neobacillus endophyticus sp. nov., an endophytic bacterium isolated from
Selaginella involvens roots. Int. J. Syst. Evol. Microbiol.. Online publication.https://doi.org/10.1099/ijsem.0.004581.

Jiang, L., Seo, J., Peng, Y., Jeon, D., Lee, J. H., Kim, C. Y. et al. 2023. A nostoxanthin-producing bacterium,
Sphingomonas nostoxan-thinifaciens sp. nov., alleviates the salt stress of
Arabidopsis seed-lings by scavenging of reactive oxygen species.
Front. Microbiol. 14: 1101150.



Joo, S. S., Jang, S. K., Kim, S. G., Choi, J. S., Hwang, K. W. and Lee, D. I. 2008. Anti-acne activity of Selaginella involvens extract and its non-antibiotic antimicrobial potential on. Propionibacterium acnes. Phytother. Res. 22: 335-339.
Katsuyama, Y. and Ohnishi, Y. 2012. Type III polyketide synthases in microorganisms.
Methods Enzymol. 515: 359-377.


Kaur, N., Chahal, K. K., Kumar, A., Singh, R. and Bhardwaj, U. 2019. Antioxidant activity of
Anethum graveolens L. essential oil con-stituents and their chemical analogues.
J. Food Biochem. 43: e12782.


Kim, D. R. and Kwak, Y. S. 2021. A genome-wide analysis of antibiotic producing genes in
Streptomyces globisporus SP6C4.
Plant Pathol. J. 37: 389-395.



Knappe, T. A., Linne, U., Zirah, S., Rebuffat, S., Xie, X. and Marahiel, M. A. 2008. Isolation and structural characterization of capistruin, a lasso peptide predicted from the genome sequence of
Burk-holderia thailandensis E264.
J. Am. Chem. Soc. 130: 11446-11454.


Komaki, H., Hosoyama, A., Yabe, S., Yokota, A., Uchino, Y. and Takano, H. 2016. Draft genome sequence of Thermogemmatispora oni-kobensis NBRC 111776T, an aerial mycelium-and spore-forming thermophilic bacterium belonging to the class. Ktedonobacteria. Genome Announc. 4: e01156-16.
Kumar, A., Singh, S., Gaurav, A. K., Srivastava, S. and Verma, J. P. 2020. Plant growth-promoting bacteria: biological tools for the miti-gation of salinity stress in plants.
Front. Microbiol. 11: 1216.



Lee, I., Kim, Y. O., Park, S. C. and Chun, J. 2016. OrthoANI: an im-proved algorithm and software for calculating average nucleo-tide identity.
Int. J. Syst. Evol. Microbiol. 66: 1100-1103.


Lei, J., Zhu, L., Zheng, Y., Yu, M., Li, G., Zhang, F. et al. 2021. Homog-enate-ultrasound-assisted ionic liquid extraction of total flavonoids from
Selaginella involven: process optimization, com-position identification, and antioxidant activity.
ACS Omega 6: 14327-14340.



Li, H., Liang, J., Chen, H., Ding, G., Ma, B. and He, N. 2016. Evolution-ary and functional analysis of mulberry type III polyketide synthases.
BMC Genomics 17: 540.



Li, S. J., Zhang, X., Wang, X. H. and Zhao, C. Q. 2018. Novel natural compounds from endophytic fungi with anticancer activity.
Eur. J. Med. Chem. 156: 316-343.


Li, W., Tang, G. H. and Yin, S. 2021. Selaginellins from the genus
Selaginella: isolation, structure, biological activity, and synthesis.
Nat. Prod. Rep. 38: 822-842.


Petrović, J., Stojković, D. and Soković, M. 2019. Terpene core in selected aromatic and edible plants: natural health improving agents.
Adv. Food Nutr. Res. 90: 423-451.

Qadri, M., Nalli, Y., Jain, S. K., Chaubey, A., Ali, A., Strobel, G. A. et al. 2017. An insight into the secondary metabolism of
Muscodor yucatanensis: small-molecule epigenetic modifiers induce expression of secondary metabolism-related genes and production of new metabolites in the endophyte.
Microb. Ecol. 73: 954-965.


Qiu, D., Wen, Y., Xie, Y. and Lin, Q. 2020. The complete chloroplast genome sequence of medicinal plant,
Selaginella involvens.
Mi-tochondrial DNA B Resour. 5: 2605-2606.



Rahimi, T., Niazi, A., Deihimi, T., Taghavi, S. M., Ayatollahi, S. and Ebra-himie, E. 2018. Genome annotation and comparative genomic analysis of
Bacillus subtilis MJ01, a new bio-degradation strain isolated from oil-contaminated soil.
Funct. Integr. Genomics 18: 533-543.


Schnitzler, J. P., Louis, S., Behnke, K. and Loivamäki, M. 2010. Poplar volatiles: biosynthesis, regulation and (eco)physiology of iso-prene and stress-induced isoprenoids.
Plant Biol. (Stuttg.) 12: 302-316.


Setyawan, A. D. and Darusman, L. K. 2008. Biflavonoid compounds of
Selaginella Pal. Beauv. andits benefit.
Biodiversitas 9: 64-81.

Somashekaraiah, R., Mottawea, W., Gunduraj, A., Joshi, U., Ham-mami, R. and Sreenivasa, M. Y. 2021. Probiotic and antifungal attributes of
Levilactobacillus brevis MYSN105, isolated from an Indian traditional fermented food pozha.
Front. Microbiol. 12: 696267.



Stahl, W. and Sies, H. 2005. Bioactivity and protective effects of natural carotenoids.
Biochim. Biophys Acta 1740: 101-107.


Sunita, K., Mishra, I., Mishra, J., Prakash, J. and Arora, N. K. 2020. Sec-ondary metabolites from halotolerant plant growth promoting rhizobacteria for ameliorating salinity stress in plants.
Front. Microbiol. 11: 567768.



Tenea, G. N. and Ortega, C. 2021. Genome characterization of
Lactiplantibacillus plantarum strain UTNGt2 originated from
Theobroma grandiflorum (white cacao) of Ecuadorian Amazon: antimicrobial peptides from safety to potential applications.
Antibiotics (Basel) 10: 383.



Wang, F. W., Ye, Y. H., Chen, J. R., Wang, X. T., Zhu, H. L., Song, Y. C. et al. 2006. Neoplaether, a new cytotoxic and antifungal endophyte metabolite from
Neoplaconema napellum IFB-E016.
FEMS Microbiol. Lett. 261: 218-223.


Woo, S., Chae, H. S., Kim, J. and Chin, Y. W. 2021. Selaginellin derivatives from
Selaginella tamariscina and their upregulating effects on low-density lipoprotein receptor expression.
J. Nat. Prod. 84: 857-864.


Zhao, J., Shan, T., Mou, Y. and Zhou, L. 2011. Plant-derived bioactive compounds produced by endophytic fungi.
Mini Rev. Med. Chem. 11: 159-168.