|
 |
| Res. Plant Dis > Volume 29(3); 2023 > Article |
|
ABSTRACT
The phenomena of global warming has led to an increase in the average air temperature in temperate climates. Springtime frost damage is becoming more common, and after a period of dormancy, damage to buds, blooms, and developing fruits is greater significant than damage from low winter temperatures. Peaches are a crucial crop among moderate fruits. Spring frost damage in peaches can have a negative effect on crop growth, yield, and quality. It is noteworthy that these plants have evolved defenses against spring frost damage while being exposed to a variety of low temperatures in the early spring. In this current review, recent research advancements on spring frost damage avoidance in peaches were deliberated. Additionally, adaptive mechanisms of peach, such as deacclimation and reacclimation, were emphasized. Moreover, the emerging advancements using various omics approaches revealed the peach physiology and molecular mechanisms comprehensively. Furthermore, the use of chemical products and understanding the spring frost mechanisms through the use of environmental chamber temperature stimulation and infrared thermography studies were also discussed. This review is essential groundwork and paves the way to derive and design future research for agronomists and horticulturalists to overcome the challenges of spring frost damage avoidance and crop management in these circumstances.
Due to abnormal climate conditions caused by global warming, air temperatures have been increasing worldwide. Over the past 50 years, the earth's temperature has climbed by 0.9°C, and it is expected to continue to do so (Lu et al., 2020). Consequently, irregular temperature changes, both high and low, have been occurring frequently all over the globe. This climate change affects various industries, including agriculture and it further causes variations in the phenol-ogy of plants. A meteorological occurrence known as spring frost (often referred as late frost or late spring frost) arises during the spring season as a result of a sudden drop in temperature to freezing or almost freezing levels after the trees have begun to bud or blossom. Their buds, blooming flowers, young fruitlets, and fruit set can be severely impacted by this occurrence, leading in a low yield and severe economic losses for farmers (Chen et al., 2016; Lee et al., 2021; Li et al., 2016). In addition, the frost tolerance of flower buds is vul-nerable as the phenological stage increases (Spiers, 1978). Therefore, flower buds that have already lost their tolerance can undergo more damage by sudden low temperatures in coming late spring.
The average temperature of early spring (February and March), when plant growth resumes, has increased by ∼2-4°C in the last 10 years (Park and Shin, 2022). The temperature rises in this period advances flowering date by inducing premature deacclimation (DA) of temperature fruit trees. According to field practices, the peach flowering date has advanced by 11.1 days over the past three decades (Lee et al., 2021; Li et al., 2016), whereas the apple flowering date has advanced by 2.7 days each decade over the past 20-25 years (Eccel et al., 2009). This scenario clearly depicts that temperate fruit trees, particularly peaches might be severely exposed to spring frost conditions for a long period of time.
While these changes occur, plants activate adaptive and defensive pathways as survival strategies. Plants develop a cold acclimation (CA) mechanism that improves their tolerance during exposure to low-temperature (Miura and Furumoto, 2013). DA, the opposite phenomenon of CA, is driven mainly by warm temperatures and is a process of losing tolerance (Pagter and Arora, 2013; Pagter et al., 2017). This phenological phenomenon fluctuates strongly and is influenced by changes in environmental factors such as low or warm temperatures (Drogoudi et al., 2020).
Peach (Prunus persica Batch) is a member of the Rosaceae family and the third temperate fruit. Peaches are among the most economically significant, nutrient-rich temperate fruits in terms of farmed surface area, right behind the apple and pear. Although China is the primary center of diversity, it is widely dispersed throughout the entire globe. According to the Food and Agriculture Organization (http://www. fao.org/faostat/en/#data/QC; accessed on August 3, 2023) report, China is the leading nectarine producer, whereas South Korea produces about 192,094 metric tons of peaches annually (Food and Agriculture Organization, 2021). In addition, peaches are a crucial model plant in the Rosaceae family because they have access to a wide range of genomic resources and genetic viewpoints that are beginning to influ-ence the evolution of new cultivars (Arús et al., 2012; Byrne et al., 2012; Muthuramalingam et al., 2022). Due to recent frost damage to temperature-sensitive fruit trees, the productivity has been reduced (Chen et al., 2023; Lee et al., 2021; Muthuramalingam et al., 2022; Wolfe et al., 2018). Indeed, poor production of peach (P. persica), apricot (P. armeniaca L.), and sweet cherry (P. avium L.) production is detrimental to farmers and consumers. Therefore, preparations for countermea-sures should be made to address issues with frost damage.
In addition, a variety of passive and active methods exist to minimize the harmful effects of frost. For instance, wind ma-chines, heaters, real-time frost warming systems, and sprin-klers belong to active methods (Ribeiro et al., 2006; Snyder and Davis, 2000; Wisniewski et al., 2016; Wolfe et al., 2018). Although these methods can have quick effects, they have disadvantages, such as being costly and having a limited range of protection. Moreover, they can expose us to haz-ards such as noise, fire, and environmental pollution. The use of appropriate cultivars, covering plants, and the application of chemical products are examples of passive approaches that, in contrast, are preemptive and have relatively long-lasting benefits (Liu and Sherif, 2019). Additionally, the selection of appropriate sites for new plantings, using cultural and management approaches, and altering the crop's physical environment are all crucial passive methods for preventing or minimizing frost damage (Ribeiro et al., 2006; Wolfe et al., 2018). To use these methods, knowledge of the plant metabolism in response to low-temperature and chemical information must be possessed. Additionally, an investigation of the reproducibility of the effects produced by using these methods is demanded. Apart from active and passive methods, there are several advanced techniques that can be used to prevent frost damage in peaches.
A mild spring frost can actually help with natural fruitlet thinning, which is important for many peach cultivars due to their high flower density, whereas a late spring frost may completely kill a peach crop (Milatović et al., 2010; Schupp et al., 2008). There is limited research on peach tolerance to natural frost due to its unpredictable nature. Based on the survival of ovaries in recently bloomed flowers, evaluation of 17 peach/nectarine and 8 Japanese plum cultivars following a natural frost at a temperature of about -3 ° C revealed significant genotype-to-genotype variation in frost tolerance during bloom. The peach cultivars Junegold and Coronet, as well as the plum cultivars Bruce and Santa Rosa, are among those that are most tolerant of frost. On the other hand, the plum cultivar Ozark Premier, the nectarine cultivar Fantasia, and the peach cultivar Loring were among the cultivars least susceptible to frost (Rieger et al., 1991).
Consequently, more efforts are needed to seek solutions that can adequately address complex aspects such as prevention effect, environment, cost, space constraints, danger-ousness, reproducibility, etc. in the current situation where the risk of frost damage to major temperate fruit trees is gradually increasing. In this review, we have discussed recent research advancements of the current spring frost protection techniques that are being used to understand the mechanism of freezing tolerance in peach plants. In addition, this review also delineates the treatment technology to minimize or prevent spring frost damage to whole peach trees.
According to Johnson and Howell (1981), freezing injury in temperate fruit trees is caused by exposure to extremely low temperatures during the winter or by spring frost after the trees have broken dormancy. Most fruit trees from temperate regions for winter survival increase their freezing tolerance by gradual exposure to low temperatures in autumn, a phenomenon known as CA (Thomashow, 1999). As opposed to CA, DA is a loss of acclimated freezing tolerance due to warm temperatures, phenological changes, and reactivation of growth (Kalberer et al., 2006). After chilling requirements have generally been met, they undergo DA and lose their freezing tolerance in spring (Weiser, 1970). Reacclimation (RA) is a process that deacclimated plants, re-exposed sub-sequently to cold temperatures, regain some or most of the lost freezing tolerance (Kalberer et al., 2006). However, due to recent irregular temperature patterns, abrupt increases in temperature during DA, driven mainly by temperature, can drastically decrease freezing tolerance within a few days (Saxe et al., 2001) and the subsequent low temperature could be lethal. Therefore, for winter and early spring survival, freezing-tolerant trees’ capacities to withstand DA during transient warm spells and to RA as low temperatures return are crucial (Kalberer et al., 2006). Additionally, mild springtime temperatures can promote budburst and ontogenetic growth, and once this development starts, tissues are unable to regain their ability to respond to cold temperatures (Pagter and Arora, 2013; Saxe et al., 2001). Temperate fruit trees should ideally be deacclimated as slowly or as late as possible during periods of unseasonably warm spring weather in order to minimize the danger of frost harm (Rowland et al., 2005). Therefore, modern cultivars must not only be exceptionally robust to extremely low temperatures but also flexible to unforeseen changes in temperature or be able to avoid unpredictable temperature patterns by going into deeper and longer dormancy. The process by which trees lose or regain their freezing tolerance in the late winter and early spring, however, is still largely unknown.
The physiological, biochemical, and molecular mechanisms of CA have been extensively studied (Thomashow, 1998; Welling and Palva, 2006; Wisniewski et al., 2003), whereas less is known about DA and/or RA (Kalberer et al., 2006; Pagter and Arora, 2013). Relatively more studies on protein changes during CA, DA, and RA have tended to be more quantitative and/or qualitative.
It is known that modifications in the carbohydrate content and the activation of associated genes protect membranes against damage brought on by freezing. It is well known that the concentration of soluble carbohydrates fluctuates in temperate perennials as they acclimate to the cold. During this time, the content of free sugars in the tissues increases by many times as starch is converted into soluble sugars (Parker, 1963). In most woody plants, sucrose is the most prevalent and often accumulated free sugar in response to low temperature (Guy et al., 1980). However, one of the most prevalent sugars in peaches is sorbitol (González-Rossia et al., 2008). Some woody plants commonly acquire lower levels of glucose and fructose (Améglio et al., 2004; Wong et al., 2003). Additionally, it has been noted that the signal transduction system in plants is impacted by sucrose and glucose (Anderson et al., 2005). Most investigations on DA have found a link between rising air temperatures, changes in freezing tolerance, and soluble carbohydrates (Kasuga et al., 2007; Morin et al., 2007; Pagter et al., 2011). Among the many enzymes and genes involved in carbohydrate metabolism, β-amylase triggered by low temperature is mostly engaged in the breakdown of starch in plants (Kossmann and Lloyd, 2000). β-amylase expression was strongly related to changes in seasonal cold hardiness in blueberry and peach (Lee et al., 2012; Shin, 2013). Furthermore, repetitive down-and up-patterns of β-amylase expression were seen in response to repeated DA and RA in peaches (Shin, 2013). Additionally, because changes in soluble sugars were clearly associated with DA (Morin et al., 2007; Pagter et al., 2011), sucrose metabolism-related enzymes like sucrose synthase and/or invertase in conjunction with β-amylase would be suitable for gene expression studies.
According to Anchordoguy et al. (1987), some temperate fruit trees have defense mechanisms that include the capacity to synthesize the proteins that protect cellular components (such as enzymes and structural proteins) from the drying effects brought on by temperature extremes and water scarcity. Storage proteins build up in huge amounts in the autumn and start to break down as spring growth begins (Wisniewski et al., 2004). Although the accurate function of dehydrins has not been verified yet, they have been accepted as one of the most important proteins related to CA and DA (Allagulova et al., 2003; Close, 1996, 1997; Marian et al., 2004; Szlachtowska and Rurek, 2023). Dehydrins are hypothetical proteins that operate as stress-response media-tors in plant defense mechanisms against salinity, drought, low temperature, and dehydration (Allagulova et al., 2003; Close, 1996, 1997; Marian et al., 2004; Szlachtowska and Rurek, 2023). In addition, dehydrins are divided into groups based on structural traits, such as the presence of conserved sequences known as the Y, S, and K segments (Allagulova et al., 2003). One class of proteins, the dehydrins, is especially linked to qualitative and quantitative variations in seasonal CA and DA (Arora and Wisniewski, 1994; Arora et al., 1992; Wisniewski et al., 2006). Several plant species have been found to contain and exhibit cryoprotective properties (Wisniewski et al., 1999; Zhang et al., 2011). The number of barks dehydrins in Hydrangea paniculata decreased during DA, but this was preceded by a large rise in bark water content (Pagter et al., unpublished data). They proposed that during DA, rehydration might serve as a stimulus for the planned dis-persion of proteins related to desiccation, such as dehydrins (Pagter and Arora, 2013). Recent studies established that DA and RA treatments simulating erratic temperature patterns due to climate change were strongly correlated with expression of dehydrin proteins in azalea and peach (Kalberer et al., 2007; Shin, 2013) and dehydrin genes (PpDhn1, PpDhn2, and PpDhn3) in peach (Shin, 2013).
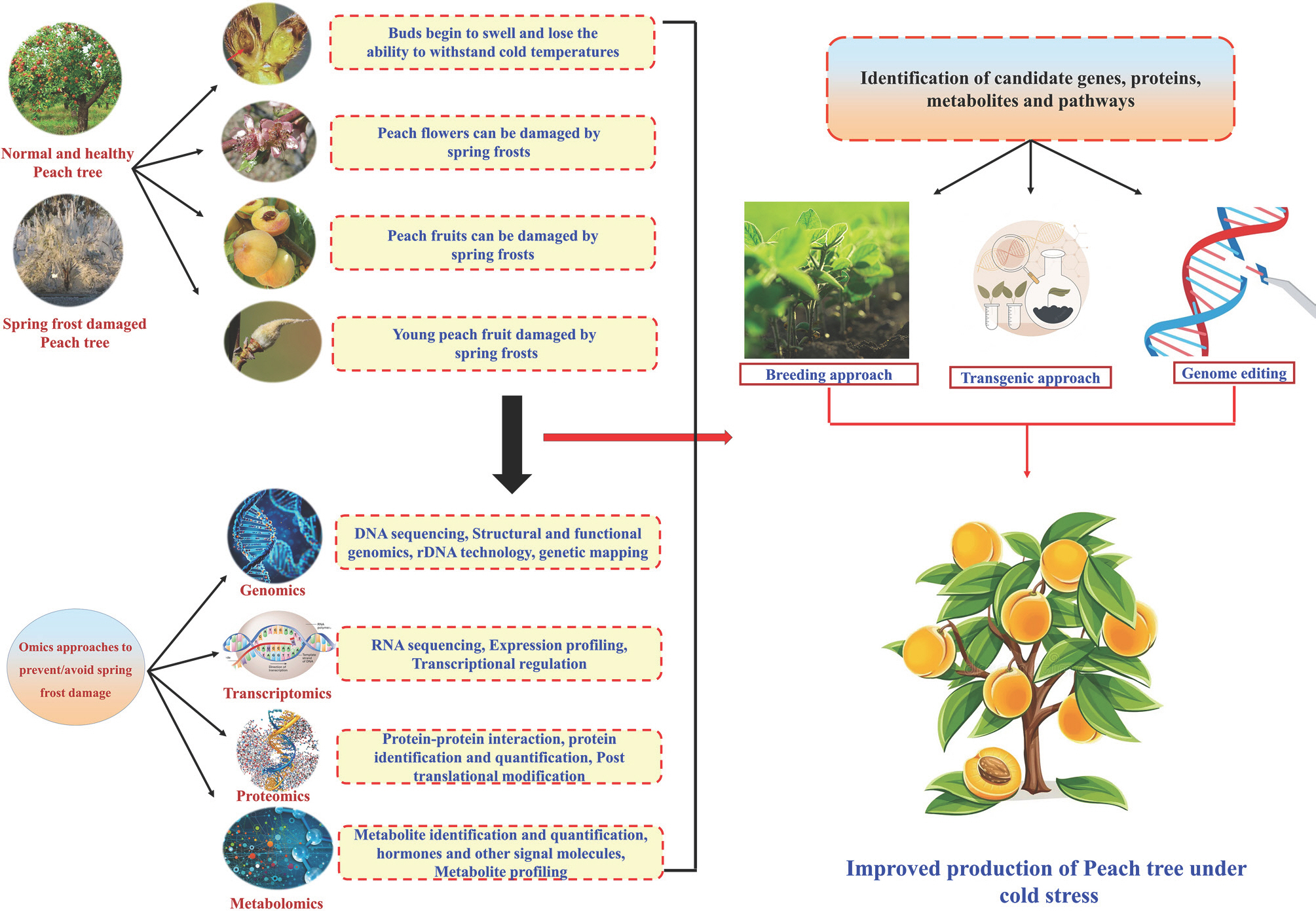
In recent years, drastic climatic changes have made temperature fluctuations, a crucial factor impacting plant growth and productivity. Several studies have examined the genes that enable rice, soybean, and other food crops to adapt to their local environments. However, few studies have investigated major fruit crops, including the domesti-cated peach (Cao et al., 2016; Li et al., 2021; Obata and Fernie, 2012; Yu et al., 2020). In the field, crops must contend with a variety of temperatures that constitute a serious threat to the production of agricultural food, including high temperatures (heat stress), cold temperatures (chilling stress), and freezing temperatures (Muthuramalingam et al., 2022; Raza et al., 2019). Most importantly, chilling stress has affected many temperate flowering and fruiting species, including peaches, by causing them to bloom earlier (Fan et al., 2010). Improving plant output requires an understanding of how plants respond to environmental changes, adjust to them, and survive temperature differences. Examining the ways that plants have enhanced their stress tolerance and survival mechanisms can offer insightful information and creative ideas for creating crops that are climate-resilient, helping to reduce the harmful effects of temperature stress. Modern biotechnological technologies like omics approaches, including genomes, transcriptomics, proteomics, and metabolomics, are being employed to do this. These methods have emerged as the most promising and cutting-edge ones for increasing crop productivity and ensuring the security of the world' food supply (Yang et al., 2021). Moreover, these methods have also been used to increase the tolerance of different plant species to freezing and/or cold stress (Fig. 1).
Physiological functions, chemical makeup, biological processes, gene sequences, structures, and their functional an-notation are all revealed through genomic exploration of an organism' genome (Muthuramalingam et al., 2019). The first wave of genomics emerged in the 1970s, and next generation sequencing (NGS) has maintained this growth.
Genome sequencing has recently advanced to third-generation sequencing technologies (Xiao and Zhou, 2020). Finding the genes and their roles in responding to stress stimuli is made possible by functional genomics. With the aid of genomics and genome data available in bioinformatic repositories, researchers can now dissect various promising approaches like transcriptomics, proteomics, metabolomics, and genome engineering or genome editing (CRISPR/Cas) system to gain detailed insights into complex stress-responsive traits and develop more resilient crops that can withstand climate fluctuations. The peach genome was sequenced in 2010 and released in 2013 with the development of the sequencing techniques (International Peach Genome Initiative et al., 2013). Investigating the genes linked to various biochemical pathways, such as stress responses like freezing tolerance, can be done utilizing this sequencing information. Numerous mapping techniques have been created, including RNA-seq, conventional quantitative trait loci (QTL) mapping, and QTL-seq analysis, which can quickly discover candidate genes inside significant QTL. QTLs associated with chilling injury have been identified in multiple linkage groups with diverse levels of reliability (Hernández Mora et al., 2017). Some of the QTLS are associated with pre-dictive molecular markers that are used in breeding. Fan et al. (2010) reported that a strong QTL for chilling requirement and blooming time was found to be localized near PpDAM6 in peach. Several transcription factors (TFs) were among the potential genes, including the ethylene responsive TF4 (ERF4), three genes encoding NAC TFs, the bZIP TF (bZIP61), and the MYP TF transparent testa 2 (TT2). According to Pons et al. (2014), a different TF (TT19) may be implicated in peach cell wall composition, susceptibility to chilling, and antho-cyanin accumulation. Another effective way for locating the potential genes and their relationships to peach freezing stress tolerance is genome-wide association studies (Cao et al., 2016). Additionally, two peach promoters that are cold-in-ducible have been discovered. These promoters, Ppbec1 and Ppxero2, respectively, code for the enzymes endochitinase (C2131) and dehydrin (C254), and they are heterologously controlled at low temperatures (Tittarelli et al., 2009). Several investigations have been conducted to determine which regions of the genome are responsible for peach freezing damage. A small number of the populations produced, compared to field crops like grains and vegetables, become a limitation of tree fruit genomic investigations. Furthermore, many studies were performed before the recent release of a genome-wide single nucleotide polymorphisms array and a new version of genome mapping. However, despite these restrictions, there are still a number of inferences that can be made. Majority of studies established that LG4 includes a region that affects the tendency of fruits to develop mealiness (Calle and Wünsch, 2020; Rawandoozi et al., 2020). Similarly, LG5 has a location that influences both mealiness and inter-nal browning. Although the identified candidate genes on these loci are suggestive, but further research is necessary to tie them along with various aspects of chilling injury. Since, the number of genes present in each locus, it is also possible that other genes are involved in addition to, or rather than, the candidate genes mentioned. The genetic investigations are therefore intriguing; further study is required to connect their results to the emergence of chilling injury.
Transcriptomics is a powerful method to quantify gene expression in a specific cell or tissue. It provides insights into the regulatory pathways and candidate genes that contribute to the resilience of peach under environmental stress conditions, which can be useful for crop breeding (Niu et al., 2020). Advanced technologies such as serial analysis of gene expression and microarrays have made it possible to obtain comprehensive transcriptome information through high-throughput analysis. To evaluate differential gene expression (DGEs), RNA-seq sequencing is commonly used. A relatively newer technique called digital gene expression is used to quantify gene expression (Wang et al., 2009). RNA-seq technique is a low cost, high-throughput sequencing method for analyzing large quantities of transcriptome data.
Studies found that cold-treated fruit mesocarp tissues had DGE patterns when cold-responsive genes in peach fruit were profiled transcriptionally using DNA microarrays (Ogundiwin et al., 2008; Vizoso et al., 2009). Generally, low temperature leads to change gene expression and increase the production of metabolites that protect the cells against damage. Dehydration responsive element-binding factor 1 (DREB1) and C-repeat-binding factor (CBF), which are both involved in one of these pathways, are members of the APETALA2/ethylene-responsive (AP2/ERF) TF superfamily (Li et al., 2020). The AP2/ERF TFs bind to the C-repeat/dehydration-responsive element in the promotors of cold-regulated genes that contribute to abiotic stress tolerance (Xie et al., 2022). Moreover, the higher level of dehydrins, which are regulated by the CBF/DREB1 TFs, serves as a mark-er of cold tolerance. In peaches, dehydrin PpDhn1 is linked with low temperature tolerance, water deficiency, and short photoperiod (Wisniewski et al., 2006). Peach productivity is significantly impacted by spring frost damage, which is also a significant environmental constraint. Peach gene expression dramatically alters in response to low temperature stress in the bark tissues of peaches (Renaut et al., 2008). The CBF family of TFs, for instance, is involved in the low temperature response. Liang et al. (2013) reported that the expression of the CBF family members PpCBF1/5/6 was relatively higher during chilling injury-delaying temperature storage and then declined after transfer to shelf-life conditions. According to Wisniewski et al. (2012), transgenic ‘M.26’ apples overexpressing a peach CBF gene (T166) displayed significantly higher levels of cold tolerance compared to transgenic ‘M.26’ apples with silenced native CBF genes (T186). In addition, a novel peach CBF TF was expressed ectopically in apple (Malus domestica) resulted in increased cold hardiness and short day-induced dormancy (Artlip et al., 2014).
NGS technology has revolutionized the genomic and tran-scriptomic approaches to biology by enabling large-scale parallel sequencing at low costs (Gupta and Verma, 2019). These platforms significantly contribute to exploring molecular mechanisms in plant metabolism. For the analysis of non-model organisms, DGE tag profiling created by Illumina Inc., is a widely used and cost-efficient method for transcriptome studies in plants. Transcriptomics can assist to unravel many mysteries related to gene expression and it will be possible to understand the molecular mechanisms associated with stress responses.
Proteomics is a robust technique that enables the investigation and detection of global changes in the plant protein structure and abundance caused by developmental and environmental signals (Liu et al., 2019; Mathabe et al., 2020). The proteomic analyses of various plant species in response to cold stress have been well-studied, including watermelon, grape, wheat, winter turnip rape, cucumber, and Petunia. However, it is still unclear how the proteomics of peach trees respond to cold stress, and the identification of key proteins associated with the cold tolerance of peach trees is rarely reported. Therefore, a comprehensive understanding of the proteomic mechanisms underlying cold tolerance in peaches is crucial.
Using differential gel electrophoresis (DIGE), a study by Nilo et al. (2010) revealed numerous proteins involved in the alterations in peach metabolism during cold storage and the subsequent ripening that led to the development of chilling injury. Under low-temperature stress conditions, 2,575 differentially expressed proteins in cold-tolerant and cold-sensitive peach cultivars were found using the isobaric tags for relative and absolute quantification (iTRAQ) technique. Among the 2,575 differentially expressed proteins, 399 and 322 showed significantly differential accumulation within cold-tolerant and cold-sensitive peach cultivars, respectively, which facilitates breeding peach cultivars with enhanced cold tolerance (Li et al., 2021). Renaut et al. (2008) investigated the protein changes in the bark tissues of peaches in response to low temperatures and short photoperiods using the DIGE method. Dehydrins are important dehydration-inducible proteins that are produced under a variety of developmental processes and are induced by cold stress. Peach PpDHN1 has been reported to increase during low temperature conditions (Artlip et al., 2016). Another study reported that peach dehydrin PCA60 acts as an antifreeze protein in response to cold stress to prevent intracellular ice formation (Herman et al., 2006). The findings revealed that the proteins subjected to significant alterations were linked to responses to stress carbohydrate, hormone production, and cellular metabolism and suggesting that thaumatin-like proteins play a protective role in maintaining the structure of cell walls against freezing injury. Additionally, according to other studies, peaches elevated in thaumatin-like proteins during storage (Dagar et al., 2010; Nilo et al., 2010). It has been determined that this protein may have served as an antifreeze protein in preventing chilling harm to the plants. Additionally, a total of 131,435 spectra were found using a combination of shotgun proteomics with 1D-gel SDS-PAGE (sodium dodecyl sulfate polyacrylamide gelelectrophoresis) and LC/MS-MS (liquid chromatographymass spectrometry) analysis, which could be linked with existing stress tolerance datasets to produce freeze-tolerant datasets (Nilo-Poyanco et al., 2021).
Metabolomics is an interdisciplinary field within systems biology that is involved with various functions (Arbona et al., 2013). Research has shown that metabolic networks are essential for plant growth and development, especially when several environmental conditions are present (Salam et al., 2023). As the intermediates or by-products of several enzy-matic reactions, metabolites operate as useful surrogates for tissue biochemical activity. With the aid of modern technology, it is almost possible to analyze tens to hundreds of metabolites in intricate biological samples (Patti et al., 2012). Several techniques can be used to examine the metabolic components in fruit cells such as HPLC (high performance liquid chromatography), UPLC (ultra-high-performance liq-uid chromatography), and GC-MS (gas chromatograph-mass spectrometer). Peach has been the subject of several metabolic investigations using GC-MS and derivatized materials.
Metabolomic study on chilling injury in peach exhibited the role of sugar levels, sucrose, glucose-6-phosphate, raffinose, galactinol, and membrane stabilizers such as polyamines in protection against freezing damage. A me-tabolomic study of peach branches exposed to cold stress identified a set of important cold-responsive metabolites. The identified metabolites, such as flavonoids, soluble sugars, and lignin, including galactose, manninotriose, glucose, luteolin, and coniferin, were found to play important roles in 1-year-old peach branches in response to cold. The findings concluded that the galactose metabolism, phenylpropanoid biosynthesis, and flavonoids biosynthesis pathways were associated with the low temperature tolerance of peaches (Li et al., 2023).
Frost events can be highly changeable and hard to fore-cast (Lamichhane, 2021). Therefore, creating artificial conditions to mimic radiant frost injury has attracted significant interest and effort (Frederiks et al., 2012). However, the temperature of ice nucleation and the freezing pattern of the whole plant can be very different under artificial and natural field conditions (Wisniewski et al., 2014). As a result, environmental chambers may be unable to replicate the type of frost damage seen in the field (Gusta and Wisniewski, 2013). Convective freezing chambers have one of the most significant problems in that it is impossible to recreate the conditions that lead to plant radiant heat loss on cold, clear nights (Wisniewski et al., 2014). Convectively cooled plants are absent dew and ice, which results in supercooling of plants (Aston and Paton, 1973). Therefore, several attempts have been conducted to develop radiantly cooled chambers that more accurately reflect spring frost (Aston and Paton, 1973). Marcellos (1981) demonstrated a small room with a radiation sink that produced a form of frost on the surfaces of wheat plants inside the container, allowing researchers to examine how plants respond to radiation frost. The frost-producing chamber created by Phillips (1984) could not replicate a true natural frost environment because the leaf temperature dropped too quickly, which was not consistent with temperature variations in the environment. Soil-Plant-Atmosphere-Research (SPAR) units provide a natural solar radiation environment and temperature, humidity, and CO2 concentration can be precisely controlled (Reddy et al., 2001). The Soil-Fruit-Daylit-System (SFDS) chamber, according to the same manufacturer, will also be able to simulate the frost environment by meeting the radiant frost conditions, as it can use natural light and precisely control the environment.
Since infrared (IR) radiation was discovered in 1800, advances in the technology to detect and photograph IR have resulted in major improvements in a variety of scientific disciplines (Livingston, 2018). Any object that is warmer than absolute zero emanates IR radiation (Speakman and Ward, 1998). IR measuring devices acquire IR rays emitted by objects, convert them into electronic signals, and generate thermograms to obtain thermal information that is not vis-ible to the human eye (Usamentiaga et al., 2014). Therefore, IR thermography is applied in various fields such as medical/veterinary medicine and the inspection field (Bogue, 2013; Lahiri et al., 2012). In addition, IR measuring is a non-contact measurement process which makes it non-destructive to target, unlike the use of sensors that are required to be injected or inserted into the subject (Usamentiga et al., 2014) and thermography simultaneously enables temperature records across a sample (Harrap and Rands, 2021).
The latent heat produced when water phase is converted to ice is the basis for the use of thermography in plant freezing research. Thermal energy that is released as the temperature drops below freezing is known as latent heat (Kaya and Kosa, 2017; Livingston et al., 2018). Therefore, if the IR camera traces the location where heat energy is generated, it is possible to determine where the ice first occurred, and the process and speed of ice propagation (Wisniewski et al., 2008). Ceccardi et al. (1995) first reported that ice formation on the surface of the excised jojoba leaves was visualized using IR camera. However, due to the poor resolution of the camera, it was difficult to confirm the exact formation point or tissue status. In a study by Wisniewski et al. (1997), the location where freezing begins in plants was visually confirmed through freeze inoculation. In addition, when the freezing of potatoes and cauliflower was monitored, it was reported that the area where the ice started was the point where the thermocouple was used (Fuller and Wisniewski, 1998). In ryegrass, the freeze pattern confirmed that the roots were frozen first, and then the crown, lower shoots, and leaves were last frozen (Stier et al., 2003). Livingston et al. (2018) investigated the freezing of wheat under natural conditions and reported that it froze first from the oldest leaves and from the base of the plant. Although thermography has a limitation in that it is impossible to image the interior of plants, it has provided many insights into the freezing and pattern of particular tissues at the whole plants.
Chemical crop protection product research is still ongoing to find less expensive, easier to apply, and more effective replacements for the current treatments (Smith, 2019). Chemical compounds used to prevent frost damage can be separated into two groups based on their mode of action (Román-Figueroa et al., 2021). One effective method for reducing temperature stress is proper plant nutrition, which also helps plants be more tolerant to temperature stress (Waraich et al., 2012). According to several research, adding nitrogen exogenously in the form of nitric oxide increases cold tolerance and lessens damage (Pakkish and Tabatabaienia, 2016; Zhu et al., 2010). Potassium also protects against low temperatures by regulating osmotic potential, reducing electrolyte leakage, and reducing the freezing point of the cell solution (Valizadehkaji and Nikoogofter Sedghi, 2020; Webster and Ebdon, 2005). Finally, applying calcium can reduce stress-related lipid peroxidation and damage to plant membranes, raise the activity of antioxidant enzymes, and improve plant resilience to stress (Juice et al., 2006; Khan et al., 2010). A crop's ability to withstand abiotic stress might, however, be severely impacted by some differences in nutrient levels or application times (Kathpalia and Bhatla, 2018). Pseudomonas syringae promotes ice formation at high sub-zero temperatures using ice-nucleating proteins attached to bacteria's outer membrane in order to get nutrients from plants (Lindow et al., 1982). For this reason, a bactericide such as a Bordeaux mixture was used for frost damage protection (Yadollahi, 2011).
Plant growth regulators (PGRs) are defined as natural substances or synthetic compounds which can affect plant development and metabolic processes (Rademacher, 2015). The use of PGRs for freezing protection has long been con-sidered through mechanisms that decrease susceptibility to abiotic stresses or delay in flowering (Rademacher, 2015; Rieger, 1989). The application of exogenous paclobutrazol inhibits the synthesis of gibberellin and enhances freezing tolerance by accumulating proline, soluble protein, and sugars (Yang et al., 2020). Abscisic acid has been reported to be effective in drought stress accompanied by freezing stress by treating citrus trees (Melgoza et al., 2014). In addition, eth-ephon not only increases the freezing tolerance of reproductive buds but also delays flowering (Liu and Sherif, 2019).
Recently, nanotechnology has been used as an effective tool to solve various problems in the agricultural field (Usman et al., 2020). Chitosan nanoparticles which were used to increase the cold stress resistance of banana plants showed high permeability and solubility, resulting in the increased level of osmoprotectant and enhancing antioxidant enzyme activity (Wang et al., 2021). Nanocellulose has been used in many industrial fields such as cosmetics and drug coating by utilizing agricultural by-products (Lam et al., 2012). Recently, it has been used as a coating material to increase the survival and germination rate of seeds (Xu et al., 2020). This suggests the possibility that nanoparticle compounds can be effective in protecting against frost damage through environmental condition control and proper agricultural management application.
Crop growth, production, and quality for peach crops are all significantly impacted by spring frost damage. This in-depth review focuses on P. persica and how their DA and RA adaptation mechanisms by cold signaling reveal the impact of temperature. However, it is still challenging to identify the molecular and physiological pathways causing spring frost damage to peach plants. Therefore, taking measures to mitigate the risk of frost damage is necessary. Prioritizing advanced research techniques that can avoid and/or prevent the risk of frost damage in peaches is crucial. Advancements in multi-omics approaches will aid in understanding the complex spring frost machinery in natural field conditions. In addition, the use of chemical products, environmental chambers, and IR thermography analyses can be used to understand spring frost damage physiology and prevent it. By applying these techniques, farmers can help ensure an effective and profitable peach crop.
Acknowledgments
This work was carried out with the support of the “Coop-erative Research Program for Agricultural Science & Technology Development (Project No. PJ014950042023)” funded by the Rural Development Administration, Republic of Korea.
REFERENCES
Allagulova, C. R., Gimalov, F. R., Shakirova, F. M. and Vakhitov, V. A. 2003. The plant dehydrins: structure and putative functions. Biochemistry (Mosc.) 68: 945-951.

Améglio, T., Decourteix, M., Alves, G., Valentin, V., Sakr, S., Julien, J. L. et al. 2004. Temperature effects on xylem sap osmolarity in walnut trees: evidence for a vitalistic model of winter embolism repair. Tree Physiol. 24: 785-793.


Anchordoguy, T. J., Rudolph, A. S., Carpenter, J. F. and Crowe, J. H. 1987. Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology 24: 324-331.


Anderson, J. V., Gesch, R. W., Jia, Y., Chao, W. S. and Horvath, D. P. 2005. Seasonal shifts in dormancy status, carbohydrate metabolism, and related gene expression in crown buds of leafy spurge. Plant Cell Environ. 28: 1567-1578.

Arbona, V., Manzi, M., de Ollas, C. and Gómez-Cadenas, A. 2013. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 14: 4885-4911.



Arora, R. and Wisniewski, M. E. 1994. Cold acclimation in genetically related (sibling) deciduous and evergreen peach (Prunus persica [L.] Batsch). II. A 60-kilodalton bark protein in cold-acclimated tissues of peach is heat stable and related to the dehydrin family of proteins. Plant Physiol. 105: 95-101.



Arora, R., Wisniewski, M. E. and Scorza, R. 1992. Cold acclimation in genetically related (sibling) deciduous and evergreen peach (Prunus persica [L.] Batsch). 1. Seasonal changes in cold hardiness and polypeptides of bark and xylem tissue. Plant Physiol. 99: 1562-1568.



Artlip, T. S., Wisniewski, M. E., Arora, R. and Norelli, J. L. 2016. An apple rootstock overexpressing a peach CBF gene alters growth and flowering in the scion but does not impact cold hardiness or dormancy. Hortic. Res. 3: 16006.




Artlip, T. S., Wisniewski, M. E. and Norelli, J. L. 2014. Field evaluation of apple overexpressing a peach CBF gene confirms its effect on cold hardiness, dormancy, and growth. Environ. Exp. Bot. 106: 79-86.

Arús, P., Verde, I., Sosinski, B., Zhebentyayeva, T. and Abbott, A. G. 2012. The peach genome. Tree Genet. Genomes 8: 531-547.


Aston, J. M. and Paton, D. M. 1973. Frost room design for radiation frost studies in Eucalyptus. Aust. J. Bot. 21: 193-199.

Bogue, R. 2013. Sensors for condition monitoring: a review of technologies and applications. Sens. Rev. 33: 295-299.

Byrne, D. H., Raseira, M. B., Bassi, D., Piagnani, M. C., Gasic, K., Reighard, G. L. et al. 2012. Peach. In: Fruit Breeding: Handbook of Plant Breeding, Vol. 8: eds. by M. L. Badenes and D. H. Byrne, pp. 505-569. Springer, New York, USA.

Calle, A. and Wünsch, A. 2020. Multiple-population QTL mapping of maturity and fruit-quality traits reveals LG4 region as a breeding target in sweet cherry (Prunus avium L.). Hortic. Res. 7: 127.




Cao, K., Zhou, Z., Wang, Q., Guo, J., Zhao, P., Zhu, G. et al. 2016. Ge-nome-wide association study of 12 agronomic traits in peach. Nat. Commun. 7: 13246.




Ceccardi, T. L., Heath, R. L. and Ting, I. P. 1995. Low-temperature exo-therm measurement using infrared thermography. HortScience 30: 140-142.

Chen, C., Okie, W. R. and Beckman, T. G. 2016. Peach fruit set and buttoning after spring frost. HortScience 51: 816-821.

Chen, R., Wang, J., Li, Y., Song, Y., Huang, M., Feng, P. et al. 2023. Quantifying the impact of frost damage during flowering on apple yield in Shaanxi province, China. Eur. J. Agron. 142: 126642.

Close, T. J. 1996. Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. Physiol. Plant. 97: 795-803.

Close, T. J. 1997. Dehydrins: a commonality in the response of plants to dehydration and low temperature. Physiol. Plant. 100: 291-296.

Dagar, A., Friedman, H. and Lurie, S. 2010. Thaumatin-like proteins and their possible role in protection against chilling injury in peach fruit. Postharvest Biol. Technol. 57: 77-85.

Drogoudi, P., Kazantzis, K., Kunz, A. and Blanke, M. M. 2020. Effects of climate change on cherry production in Naoussa, Greece and Bonn, Germany: adaptation strategies. EuroMediterr. J. Environ. Integr. 5: 12.


Eccel, E., Rea, R., Caffarra, A. and Crisci, A. 2009. Risk of spring frost to apple production under future climate scenarios: the role of phenological acclimation. Int. J. Biometeorol. 53: 273-286.



Fan, S., Bielenberg, D. G., Zhebentyayeva, T. N., Reighard, G. L., Okie, W. R., Holland, D. et al. 2010. Mapping quantitative trait loci associated with chilling requirement, heat requirement and bloom date in peach (Prunus persica). New Phytol. 185: 917-930.



Food and Agriculture Organization (FAO). 2021. URLhttp://www.fao.org/faostat/en/#data/QC. [3 August 2023].
Frederiks, T. M., Christopher, J. T., Harvey, G. L., Sutherland, M. W. and Borrell, A. K. 2012. Current and emerging screening methods to identify post-head-emergence frost adaptation in wheat and barley. J. Exp. Bot. 63: 5405-5416.


Fuller, M. P. and Wisniewski, M. 1998. The use of infrared thermal imaging in the study of ice nucleation and freezing in plants. J. Therm. Biol. 23: 81-89.
González-Rossia, D., Reig, C., Dovis, V., Gariglio, N. and Agustí, M. 2008. Changes in carbohydrates and nitrogen content in the bark tissues induced by artificial chilling and its relationship with dormancy bud break in Prunus sp. Sci. Hortic. 118: 275-281.
Gupta, N. and Verma, V. K. 2019. Next-generation sequencing and its application: empowering in public health beyond reality. In: Microbial Technology for the Welfare of Society, ed. by P. K. Arora, pp. 313-341. Springer, Singapore.


Gusta, L. V. and Wisniewski, M. 2013. Understanding plant cold hardiness: an opinion. Physiol. Plant. 147: 4-14.


Guy, C. L., Yelenosky, G. and Sweet, H. C. 1980. Light exposure and soluble sugars in citrus frost hardiness. Fl. Sci. 43: 268-273.
Harrap, M. J. M. and Rands, S. A. 2021. Floral infrared emissivity esti-mates using simple tools. Plant Methods 17: 23.




Herman, E. M., Rotter, K., Premakumar, R., Elwinger, G., Bae, R., Ehler-King, L. et al. 2006. Additional freeze hardiness in wheat acquired by exposure to −3 o C is associated with extensive physiological, morphological, and molecular changes. J. Exp. Bot. 57: 3601-3618.

Hernández Mora, J. R., Micheletti, D., Bink, M., Van de Weg, E., Cantín, C., Nazzicari, N. et al. 2017. Integrated QTL detection for key breeding traits in multiple peach progenies. BMC Genomics 18: 404.




International Peach Genome Initiative, Verde, I., Abbott, A. G., Scalabrin, S., Jung, S., Shu, S. et al. 2013. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat. Genet. 45: 487-494.



Johnson, D. E. and Howell, G. S. 1981. Factors influencing critical temperatures for spring freeze damage to developing primary shoots on Concord grapevines. Am. J. Enol. Vitic. 32: 144-149.

Juice, S. M., Fahey, T. J., Siccama, T. G., Driscoll, C. T., Denny, E. G., Eagar, C. et al. 2006. Response of sugar maple to calcium addition to northern hardwood forest. Ecology 87: 1267-1280.


Kalberer, S. R., Leyva-Estrada, N., Krebs, S. L. and Arora, R. 2007. Frost dehardening and rehardening of floral buds of deciduous aza-leas are influenced by genotypic biogeography. Environ. Exp. Bot. 59: 264-275.

Kalberer, S. R., Wisniewski, M. and Arora, R. 2006. Deacclimation and reacclimation of cold-hardy plants: current understanding and emerging concepts. Plant Sci. 171: 3-16.

Kasuga, J., Arakawa, K. and Fujikawa, S. 2007. High accumulation of soluble sugars in deep supercooling Japanese white birch xylem parenchyma cells. New Phytol. 174: 569-579.



Kathpalia, R. and Bhatla, S. C. 2018. Plant mineral nutrition. In: Plant Physiology, Development and Metabolism, eds. by S. C. Bhatla and M. A. Lal, pp. 37-81. Springer, Singapore.

Kaya, Ö. and Köse, C. 2017. Determination of resistance to low temperatures of winter buds on lateral shoot present in Karaerik (Vitis vinifera L.) grape cultivar. Acta Physiol. Plant. 39: 209.


Khan, M. N., Siddiqui, M. H., Mohammad, F., Naeem, M. and Khan, M. M. A. 2010. Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol. Plant. 32: 121-132.


Kossmann, J. and Lloyd, J.R. 2000. Understanding and influencing starch biochemistry. Crit. Rev. Plant Sci. 19: 171-226.

Lahiri, B. B., Bagavathiappan, S., Jayakumar, T. and Philip, J. 2012. Medical applications of infrared thermography: a review. Infrared Phys. Technol. 55: 221-235.



Lam, E., Male, K. B., Chong, J. H., Leung, A. C. W. and Luong, J. H. T. 2012. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol. 30: 283-290.


Lamichhane, J. R. 2021. Rising risks of late-spring frosts in a changing climate. Nat. Clim. Change 11: 554-555.


Lee, J. H., Yu, D. J., Kim, S. J., Choi, D. and Lee, H. J. 2012. Intraspe-cies differences in cold hardiness, carbohydrate content and β-amylase gene expression of Vaccinium corymbosum during cold acclimation and deacclimation. Tree Physiol. 32: 1533-1540.


Lee, S., Jeong, J. H., Kim, S. H. and Shin, H. 2021. Freezing tolerance enhancement and thermographic observation of whole peach trees applied with cellulose nanocrystals under realistic spring frost conditions using a soil-fruit-daylit-system. Plants 10: 2301.



Li, W., Chen, Y., Ye, M., Lu, H., Wang, D. and Chen, Q. 2020. Evolution-ary history of the C-repeat binding factor/dehydration-respon-sive element-binding 1 (CBF/DREB1) protein family in 43 plant species and characterization of CBF/DREB1 proteins in Solanum tuberosum. BMC Evol. Biol. 20: 142.




Li, Y., Tian, Q., Wang, Z., Li, J., Liu, S., Chang, R. et al. 2023. Integrated analysis of transcriptomics and metabolomics of peach under cold stress. Front. Plant Sci. 14: 1153902.



Li, Y., Wang, Z., Tian, Q., Zhou, Y., Xu, J., Chang, R. et al. 2021. Quantitative proteomic analyses on the mechanisms of cold tolerance in two peach cultivars (Prunus persica L. Batsch) based on iTRAQ. Eur. J. Hortic. Sci. 86: 308-319.

Li, Y., Wang, L., Zhu, G., Fang, W., Cao, K., Chen, C. et al. 2016. Pheno-logical response of peach to climate change exhibits a relatively dramatic trend in China, 1983-2012. Sci. Hortic. 209: 192-200.

Liang, L., Zhang, B., Yin, X. R., Xu, C. J., Sun, C. D. and Chen, K. S. 2013. Differential expression of the CBF gene family during postharvest cold storage and subsequent shelf-life of peach fruit. Plant Mol. Biol. Rep. 31: 1358-1367.


Lindow, S. E., Arny, D. C. and Upper, C. D. 1982. Bacterial ice nucleation: a factor in frost injury to plants. Plant Physiol. 70: 1084-1089.



Liu, Y., Lu, S., Liu, K., Wang, S., Huang, L. and Guo, L. 2019. Proteomics: a powerful tool to study plant responses to biotic stress. Plant Methods 15: 135.




Livingston, D. P., Tuong, T. D., Murphy, J. P., Gusta, L. V., Willick, I. and Wisniewski, M. E. 2018. High-definition infrared thermography of ice nucleation and propagation in wheat under natural frost conditions and controlled freezing. Planta 247: 791-806.




Lu, H., Guan, Y., He, L., Adhikari, H., Pellikka, P., Heiskanen, J. et al. 2020. Patch aggregation trends of the global climate landscape under future global warming scenario. Int. J. Climatol. 40: 2674-2685.


Marcellos, H. 1981. A plant freezing chamber with radiative and convective energy exchange. J. Agric. Eng. Res. 26: 403-408.

Marian, C. O., Krebs, S. L. and Arora, R. 2004. Dehydrin variability among Rhododendron species: a 25-kDa dehydrin is conserved and associated with cold acclimation across diverse species. New Phytol. 161: 773-780.



Mathabe, P. M. K., Belay, Z. A., Ndlovu, T. and Caleb, O. J. 2020. Prog-ress in proteomic profiling of horticultural commodities during postharvest handling and storage: a review. Sci. Hortic. 261: 108996.

Melgoza, F. J., Kusakabe, A., Nelson, S. D. and Melgar, J. C. 2014. Ex-ogenous applications of abscisic acid increase freeze tolerance in citrus trees. Int. J. Fruit Sci. 14: 376-387.

Milatović, D., Nikolić, D. and Đurović, D. 2010. Variability, heritabil-ity and correlations of some factors affecting productivity in peach. Hortic. Sci. 37: 79-87.

Miura, K. and Furumoto, T. 2013. Cold signaling and cold response in plants. Int. J. Mol. Sci. 14: 5312-5337.



Morin, X., Améglio, T., Ahas, R., Kurz-Besson, C., Lanta, V., Lebour-geois, F. et al. 2007. Variation in cold hardiness and carbohydrate concentration from dormancy induction to bud burst among provenances of three European oak species. Tree Physiol. 27: 817-825.


Muthuramalingam, P., Jeyasri, R., Kalaiyarasi, D., Pandian, S., Krish-nan, S. R., Satish, L. et al. 2019. Emerging advances in compu-tational omics tools for systems analysis of Gramineae family grass species and their abiotic stress responsive functions. In: OMICS-Based Approach Plant Biotechnology, eds. by R. Ba-nerjee, G. V. Kumar and S. P. Jeevan Kumar, pp. 185-216. Wiley, Hoboken, NJ, USA.


Muthuramalingam, P., Shin, H., Adarshan, S., Jeyasri, R., Priya, A., Chen, J. T. et al. 2022. Molecular insights into freezing stress in peach based on multi-omics and biotechnology: an overview. Plants 11: 812.



Nilo, R., Saffie, C., Lilley, K., Baeza-Yates, R., Cambiazo, V., Campos-Vargas, R. et al. 2010. Proteomic analysis of peach fruit mesocarp softening and chilling injury using difference gel electrophoresis (DIGE). BMC Genomics 11: 43.




Nilo-Poyanco, R., Moraga, C., Benedetto, G., Orellana, A. and Almei-da, A. M. 2021. Shotgun proteomics of peach fruit reveals major metabolic pathways associated to ripening. BMC Genomics 22: 17.




Niu, R., Zhao, X., Wang, C. and Wang, F. 2020. Transcriptome profiling of Prunus persica branches reveals candidate genes poten-tially involved in freezing tolerance. Sci. Hortic. 259: 108775.

Obata, T. and Fernie, A. R. 2012. The use of metabolomics to dissect plant responses to abiotic stresses. Cell. Mol. Life Sci. 69: 3225-3243.



Ogundiwin, E. A., Marti, C., Forment, J., Pons, C., Granell, A., Gradziel, T. M. et al. 2008. Development of ChillPeach genomic tools and identification of cold-responsive genes in peach fruit. Plant Mol. Biol. 68: 379-397.



Pagter, M., Alpers, J., Erban, A., Kopka, J., Zuther, E. and Hincha, D. K. 2017. Rapid transcriptional and metabolic regulation of the deacclimation process in cold acclimated. Arabidopsis thaliana. BMC Genomics 18: 731.
Pagter, M. and Arora, R. 2013. Winter survival and deacclimation of perennials under warming climate: physiological perspectives. Physiol. Plant. 147: 75-87.


Pagter, M., Hausman, J. F. and Arora, R. 2011. Deacclimation kinetics and carbohydrate changes in stem tissues of Hydrangea in response to an experimental warm spell. Plant Sci. 180: 140-148.


Pakkish, Z. and Tabatabaienia, M. S. 2016. The use and mechanism of NO to prevent frost damage to flower of apricot. Sci. Hortic. 198: 318-325.

Park, Y. and Shin, H. 2022. Frost avoidance: sodium alginate + CaCl2 can postpone flowering of ‘Kawanakajima Hakuto’ peach trees. Hortic. Environ. Biotechnol. 63: 643-650.


Patti, G. J., Yanes, O. and Siuzdak, G. 2012. Innovation: metabolomics: the apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 13: 263-269.




Phillips, D. E. 1984. Environment test chamber. U.S. Patent H229.
Pons, C., Martí, C., Forment, J., Crisosto, C. H., Dandekar, A. M. and Granell, A. 2014. A bulk segregant gene expression analysis of a peach population reveals components of the underlying mechanism of the fruit cold response. PLoS ONE 9: e90706.



Rademacher, W. 2015. Plant growth regulators: backgrounds and uses in plant production. J. Plant Growth Regul. 34: 845-872.


Rawandoozi, Z. J., Hartmann, T. P., Carpenedo, S., Gasic, K., da Silva Linge, C., Cai, L. et al. 2020. Identification and characterization of QTLs for fruit quality traits in peach through a multi-family approach. BMC Genomics 21: 522.




Raza, A., Razzaq, A., Mehmood, S. S., Zou, X., Zhang, X., Lv, Y. et al. 2019. Impact of climate change on crops adaptation and strategies to tackle its outcome: a review. Plants 8: 34.



Reddy, K. R., Hodges, H. F., Read, J. J., McKinion, J. M., Baker, J. T., Tar-pley, L. et al. 2001. Soil-Plant-Atmosphere-Research (SPAR) facil-ity: a tool for plant research and modeling. Biotronics 30: 27-50.
Renaut, J., Hausman, J. F., Bassett, C., Artlip, T., Cauchie, H. M., Wit-ters, E. et al. 2008. Quantitative proteomic analysis of short photoperiod and low-temperature responses in bark tissues of peach (Prunus persica L. Batsch). Tree Genet. Genomes 4: 589-600.


Ribeiro, A. C., De Melo-Abreu, J. P. and Snyder, R. L. 2006. Apple or-chard frost protection with wind machine operation. Agric. For. Meteorol. 141: 71-81.

Rieger, M., Lu, S. and Duemmel, M. 1991. Frost tolerance of some peach and Japanese plum cultivars. Fruit Var. J. 45: 3-6.
Román-Figueroa, C., Bravo, L., Paneque, M., Navia, R. and Cea, M. 2021. Chemical products for crop protection against freezing stress: a review. J. Agron. Crop Sci. 207: 391-403.


Rowland, L. J., Ogden, E. L., Ehlenfeldt, M. K. and Vinyard, B. 2005. Cold hardiness, deacclimation kinetics, and bud development among 12 diverse blueberry genotypes under field conditions. J. Am. Soc. Hortic. Sci. 130: 508-514.

Salam, U., Ullah, S., Tang, Z. H., Elateeq, A. A., Khan, Y., Khan, J. et al. 2023. Plant metabolomics: an overview of the role of primary and secondary metabolites against different environmental stress factors. Life 13: 706.



Saxe, H., Cannell, M. G. R., Johnson, Ø., Ryan, M. G. and Vourlitis, G. 2001. Tree and forest functioning in response to global warming. New Phytol. 149: 369-399.



Schupp, J. R., Baugher, T. A., Miller, S. S., Harsh, R. M. and Lesser, K. M. 2008. Mechanical thinning of peach and apple trees reduces labor input and increases fruit size. HortTechnology 18: 660-670.

Shin, H. 2013. Changes of dehydrins, carbohydrates, and gene expressions related to cold hardiness in Prunus persica (L.) Batsch. Ph.D. thesis. Chungbuk National University, Cheongju, Korea.141.
Smith, E. D. 2019. Cold hardiness and options for the freeze protection of southern highbush blueberry. Agriculture 9: 9.

Snyder, R. L. and Davis, C. A. 2000. Principles of Frost Protection. Long Version-Quick Answer FP005. University of California, At-mospheric Science, Davis, CA, USA. pp. 26 pp.
Speakman, J. R. and Ward, S. 1998. Infrared thermography: prin-ciples and applications. Zoology 101: 224-232.
Spiers, J. M. 1978. Effect of stage of bud development on cold injury in rabbiteye blueberry. J. Am. Soc. Hortic. Sci. 103: 452-455.
Stier, J. C., Filiault, D. L., Wisniewski, M. and Paulta, J. P. 2003. Visualization of freezing progression in turfgrass using infrared video thermography. Crop Sci. 43: 415-420.
Szlachtowska, Z. and Rurek, M. 2023. Plant dehydrins and dehydrin-like proteins: characterization and participation in abiotic stress response. Front Plant Sci. 6(14):1213188.



Thomashow, M. F. 1998. Role of cold-responsive genes in plant freezing tolerance. Plant Physiol. 118: 1-8.


Thomashow, M. F. 1999. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50: 571-599.


Tittarelli, A., Santiago, M., Morales, A., Meisel, L. A. and Silva, H. 2009. Isolation and functional characterization of cold-regulated pro-moters, by digitally identifying peach fruit cold-induced genes from a large EST dataset. BMC Plant Biol. 9: 121.




Usamentiaga, R., Venegas, P., Guerediaga, J., Vega, L., Molleda, J. and Bulnes, F. G. 2014. Infrared thermography for temperature measurement and non-destructive testing. Sensors 14: 12305-12348.



Usman, M., Farooq, M., Wakeel, A., Nawaz, A., Cheema, S. A., ur Rehman, H. et al. 2020. Nanotechnology in agriculture: current status, challenges and future opportunities. Sci. Total Environ. 721: 137778.


ValizadehKaji, B. and Nikoogoftar Sedghi, M. 2020. Enhancement of frost tolerance of almond flowers using potassium. J. Plant Nutr. 43: 2822-2832.

Vizoso, P., Meisel, L. A., Tittarelli, A., Latorre, M., Saba, J., Caroca, R. et al. 2009. Comparative EST transcript profiling of peach fruits under different postharvest conditions reveals candidate genes associated with peach fruit quality. BMC Genomics 10: 423.




Wang, A., Li, J., Al-Huqail, A. A., Al-Harbi, M. S., Ali, E. F., Wang, J. et al. 2021. Mechanisms of chitosan nanoparticles in the regulation of cold stress resistance in banana plants. Nanomaterials 11: 2670.



Wang, Z., Gerstein, M. and Snyder, M. 2009. RNA-Seq: a revolution-ary tool for transcriptomics. Nat. Rev. Genet. 10: 57-63.




Waraich, E. A., Ahmad, R., Halim, A. and Aziz, T. 2012. Alleviation of temperature stress by nutrient management in crop plants: a review. J. Soil Sci. Plant Nutr. 12: 221-244.

Webster, D. E. and Ebdon, J. S. 2005. Effects of nitrogen and potassium fertilization on perennial ryegrass cold tolerance during deacclimation in late winter and early spring. HortScience 40: 842-849.

Weiser, C. J. 1970. Cold resistance and injury in woody plants: knowledge of hardy plant adaptations to freezing stress may help us to reduce winter damage. Science 169: 1269-1278.


Welling, A. and Palva, E. T. 2006. Molecular control of cold acclimation in trees. Physiol. Plant. 127: 167-181.

Wisniewski, M., Artlip, T. and Norelli, J. 2016. Dealing with frost damage and climate change in tree fruit crops. New York Fruit Q. 24: 25-28.
Wisniewski, M., Bassett, C. and Arora, R. 2004. Distribution and partial characterization of seasonally expressed proteins in different aged shoots and roots of ‘Loring’ peach (Prunus persica). Tree Physiol. 24: 339-345.


Wisniewski, M. E., Bassett, C. and Gusta, L. V. 2003. An overview of cold hardiness in woody plants: seeing the forest through the trees. HortScience 38: 952-959.

Wisniewski, M. E., Bassett, C. L., Renaut, J., Farrell, R. Jr., Tworkoski, T. and Artlip, T. S. 2006. Differential regulation of two dehydrin genes from peach (Prunus persica) by photoperiod, low temperature and water deficit. Tree Physiol. 26: 575-584.


Wisniewski, M., Glenn, D. M., Gusta, L. and Fuller, M. P. 2008. Using infrared thermography to study freezing in plants. HortScience 43: 1648-1651.

Wisniewski, M., Gusta, L. and Neuner, G. 2014. Adaptive mechanisms of freeze avoidance in plants: a brief update. Environ. Exp. Bot. 99: 133-140.

Wisniewski, M., Lindow, S. E. and Ashworth, E. N. 1997. Observations of ice nucleation and propagation in plants using infrared video thermography. Plant Physiol. 113: 327-334.




Wisniewski, M. E., Norelli, J. L., Phillips, J. G., Artlip, T. S. and Korban, S. 2012. Using an apple microarray to characterize the CBF-regu-lon in transgenic ‘M.26’ apple trees overexpressing a peach CBF gene. ASHS National Conference Program. pp. 45American Society for Horticultural Science, Alexandria, VA, USA.
Wisniewski, M. E., Webb, R., Balsamo, R., Close, T. J., Yu, X. M. and Griffith, M. 1999. Purification, immunolocalization, cryoprotective, and antifreeze activity of PCA60: a dehydrin from peach (Prunus persica). Physiol. Plant. 105: 600-608.


Wolfe, D. W., DeGaetano, A. T., Peck, G. M., Carey, M., Ziska, L. H., Lea-Cox, J. et al. 2018. Unique challenges and opportunities for northeastern US crop production in a changing climate. Clim. Change 146: 231-245.


Wong, B. L., Baggett, K. L. and Rye, A. H. 2003. Seasonal patterns of reserve and soluble carbohydrates in mature sugar maple (Acer saccharum). Can. J. Bot. 81: 780-788.

Xiao, T. and Zhou, W. 2020. The third generation sequencing: the advanced approach to genetic diseases. Transl. Pediatr. 9: 163-173.



Xie, Z., Yang, C., Liu, S., Li, M., Gu, L., Peng, X. et al. 2022. Identification of AP2/ERF transcription factors in Tetrastigma hemsleyan-um revealed the specific roles of ERF46 under cold stress. Front. Plant Sci. 13: 936602.



Xu, T., Ma, C., Aytac, Z., Hu, X., Ng, K. W., White, J. C. et al. 2020. En-hancing agrichemical delivery and seedling development with biodegradable, tunable, biopolymer-based nanofiber seed coatings. ACS Sustain. Chem. Eng. 8: 9537-9548.

Yadollahi, A. 2011. Evaluation of reduction approaches on frost damages of grapes grown in moderate cold climate. Afr. J. Agric. Res. 6: 6289-6295.
Yang, C., Duan, W., Xi, K., Ren, C., Zhu, C., Chen, K. et al. 2020. Effect of salicylic acid treatment on sensory quality, flavor-related chemicals and gene expression in peach fruit after cold storage. Postharvest Biol. Technol. 161: 111089.

Yang, Y., Saand, M. A., Huang, L., Abdelaal, W. B., Zhang, J., Wu, Y. et al. 2021. Applications of multi-omics technologies for crop im-provement. Front. Plant Sci. 12: 563953.



Yang, Y., Zhang, R., Duan, X., Hu, Z., Shen, M. and Leng, P. 2019. Natural cold acclimation of Ligustrum lucidum in response to exogenous application of paclobutrazol in Beijing. Acta Physiol. Plant. 41: 15.


Yu, D. J., Jun, S. H., Park, J., Kwon, J. H. and Lee, H. J. 2020. Transcriptome analysis of genes involved in cold hardiness of peach tree (Prunus persica) shoots during cold acclimation and deacclimation. Genes 11: 611.



Zhang, X., Wang, K., Ervin, E. H., Waltz, C. and Murphy, T. 2011. Meta-bolic changes during cold acclimation and deacclimation in five bermudagrass varieties. 1. Proline, total amino acid, protein, and dehydrin expression. Crop Sci. 51: 838-846.
- TOOLS
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 1,424 View
- 47 Download
- ORCID iDs
-
Pandiyan Muthuramalingam

https://orcid.org/0000-0001-7294-1625Yeonju Park

https://orcid.org/0009-0004-1280-373XHyunsuk Shin

https://orcid.org/0000-0002-2167-4843 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print