Occurrence of Gray Mold in Mango Caused by Botrytis cinerea in Korea
Article information
Abstract
During a disease survey conducted in April 2022, we observed severe gray mold symptoms on inflorescences of mango trees (Mangifera indica) grown in a vinyl greenhouse in Jeju, Korea. The symptoms occurred on the flowers and peduncles, on which a lot of gray molds were formed. The incidence of gray mold on the inflorescences in the vinyl greenhouse ranged from 10% to 40%. Three fungal isolates were obtained from the lesions and identified as Botrytis cinerea based on their morphological characteristics and phylogenetic analysis. All isolates of B. cinerea were tested for their pathogenicity to inflorescences of mango trees through artificial inoculation. The pathogenicity of the isolates was confirmed on the flowers and peduncles. The symptoms induced by the isolates were similar to those on the inflorescences of mango trees observed in the disease survey. This is the first report of B. cinerea causing gray mold in mango in Korea.
Mango (Mangifera indica) is a tree belonging to the family Anacardiaceae, and its native is Assam, Belize, China South-Central, East Himalaya, Myanmar, and Thailand (Plants of the World Online, 2023). The tree grows primarily in the tropical and subtropical zones. In Korea, the tree has been mostly cultivated as a fruit tree in vinyl greenhouses in Jeju area. During a disease survey conducted in April 2022, we observed severe gray mold symptoms on inflorescences of mango trees grown in a vinyl greenhouse in Jeju, Korea. The symptoms occurred on the flowers and peduncles, on which a lot of gray molds were formed (Fig. 1A, B). The diseased flowers turned brown and blighted, and the diseased peduncles turned brown to black and rotted. As the disease progressed, black and long lesions were formed on the axes of inflorescences. In the vinyl greenhouse, five trees were observed, and 100 inflorescences of each tree were investigated for incidence of gray mold. The disease incidence on the inflorescences in the vinyl greenhouse ranged from 10% to 40%.

Gray mold symptoms of mango trees. (A, B) Symptoms on the flowers and peduncles observed in the vinyl greenhouse. (C) Symptoms on the flowers and peduncles induced by artificial inoculation test with Botrytis cinerea isolate (MZBC-2202). (D) A non-inoculated tree (control).
Mango inflorescences with gray mold symptoms were collected from the investigated vinyl greenhouse, and fungi were isolated from the diseased inflorescences. Conidial suspension was prepared from conidial masses on the gray mold symptoms using sterile distilled water and streaked on 2% water agar (WA) plate using a sterile loop. After incubation of the WA plate for 24 hr at 20 ° C, germinated conidia on WA were picked up under a dissecting microscope (Nikon SMZ 1780, Tokyo, Japan) and transferred to new WA plates. Three single-conidium isolates were obtained from the WA plate cultures after 5 days of incubation at 20 ° C. The isolates were cultured in potato dextrose agar (PDA) slants to use for identification and pathogenicity tests.
The three fungal isolates (MZBC-2201, MZBC-2202, and MZBC-2203) were cultured on PDA in 9 cm diameter Petri dishes at 20°C in the dark for 25 days to investigate their cul-tural and morphological characteristics. The colonies of the isolates cultured on PDA consisted of white to gray mycelia and black, spherical or irregular sclerotia of which diameter was 1‒7 mm (Fig. 2A). Fifty conidia and 25 conidiophores of each isolate were examined for their morphology. Conidia were hyaline to pale brown, globose or ellipsoidal, and measured 7‒14×5‒11 μm (av. 10.6×8.4 μm) (Fig. 2B). Conid-iophores were erect, long more than 1 mm, 13‒18 μm thick, branched, brown below, and paler near the apex. The morphological characteristics of the isolates were similar to those of Botrytis cinerea described in a previous study (Ellis, 1971).

Cultural and morphological features of Botrytis cinerea isolates from diseased mango trees. (A) A colony of an isolate (MZBC-2202) grown on potato dextrose agar at 20°C for 25 days. (B) A conidiophore and conidia.
Phylogenetic analysis was conducted to confirm the identification of B. cinerea isolates based on their morphological characteristics. Genomic DNA of the isolates was extracted using the protocol in a previous study (Dong et al., 2022), with slight modifications. Polymerase chain reaction (PCR) products of glyceraldehyde-3-phosphate dehydrogenase (G3PDH), heat-shock protein 60 (HSP60), and DNA-depen-dent RNA polymerase subunit II (RPB2) gene regions were obtained using the primer sets with the amplification conditions described in a previous study (Staats et al., 2005) and DNA Free-Multiplex Master Mix (Cellsafe, Yongin, Korea). Pu-rification of the PCR products was done using the universal DNA purification kit (Tiangen, Beijing, China). Sequencing of the PCR products was conducted at Bionics Co., Ltd. (Seoul, Korea) with the same primers. The sequences were adjusted by SeqMan II (DNASTAR Inc., Madison, WI, USA) if necessary. Alignment of the sequences of the isolates from mango and other Botrytis spp. (Staats et al., 2005) was done using MUSCLE (Edgar, 2004). Sclerotinia sclerotiorum 484 (Van Der Vlugt-Bergmans, 1993) was selected as an outgroup taxon. MEGA version 7 software (Kumar et al., 2016) was used to process and enhance the multiple sequence alignments, if necessary. Neighbor-joining analysis for concatenated alignments was conducted with maximum composite likelihood model performing 1,000 bootstrap replicates by MEGA version 7 software (Kumar et al., 2016). The phylogenetic analysis revealed that all isolates were clustered with the two strains (BC7 b and MUCL87) of B. cinerea (Fig. 3). The sequence data of G3PDH, HSP60, and RPB2 genes obtained from the three isolates were deposited in GenBank with accession num-bers of OR469305‒ OR469307, OR469308‒ OR469310, and OR469311‒ OR469313, respectively.
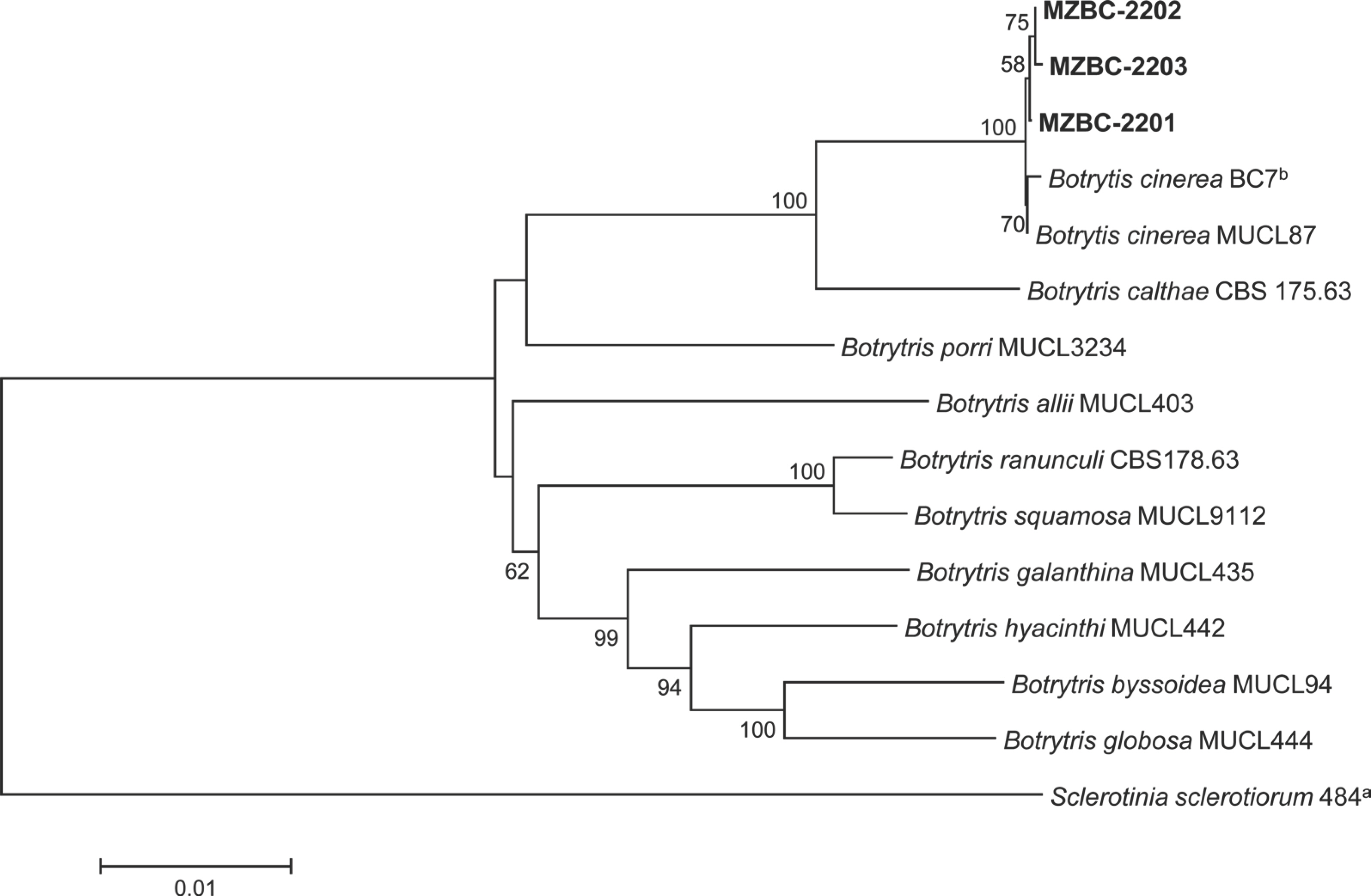
Phylogenetic tree based on glyceraldehyde-3-phosphate dehydrogenase, heat-shock protein 60, and DNA-dependent RNA polymerase subunit II gene regions of the three isolates (MZBC-2201, MZBC-2202, and MZBC-2203) from mango and reference strains of Botrytis species. Sequence data of the reference species were obtained from the NCBI GenBank database. The phylogenetic tree was generated using the neighbor-joining method with a maximum composite likelihood model. The bootstrap support values more than 50 are shown at the nodes. The scale bar represents the number of nucleotide substitutions per site.
The three isolates of B. cinerea were tested for their pathogenicity to inflorescences of mango trees through artificial inoculation. A conidial suspension (1‒2×106 conidia/ml) of each isolate was prepared from the 30-day-old PDA cultures. A 40 ml of the conidial suspension of each isolate was sprayed onto inflorescences of a three-year-old mango tree grown in a circular plastic pot (height: 30 cm; diameter: 32 cm) in a vinyl greenhouse. A control mango tree was treated with 40 ml of sterile distilled water. The inoculated trees were covered with plastic bags of which inside was wetted with sterile distilled water and placed in a cultivation room at 18‒22°C for 7 days. Thereafter, the plastic bags were removed from the inoculated trees, and the inoculated trees were placed in the cultivation room. Pathogenicity of the isolates was rated based on the de-gree of gray mold symptoms 10 days after inoculation. The inoculation test was conducted in three replicates. All the tested isolates caused gray mold symptoms on the flowers and peduncles of the inoculated trees (Fig. 1C), but no symptoms were observed on those of the control trees (Fig. 1D). The symptoms induced by the isolates were similar to those on the inflorescences of mango trees observed in the disease survey. Re-isolation of the inoculated isolates from the lesions was confirmed.
B. cinerea is known as a cosmopolitan gray mold and damages flowers, leaves, stems, fruits, etc. of many plants (Ellis, 1971). It has been reported that B. cinerea is the causal agent of gray mold on floral tissue of mango in Japan (Ajitomi et al., 2022) and causes stem end rot of mango fruit in Pakistan (Alam et al., 2017). The disease occurrence in many plants was reported in Korea (Korean Society of Plant Pathology, 2023). However, there has been no report on the disease occurrence in mango. This is the first report of B. cinerea causing gray mold in mango in Korea.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
This study was supported by a research grant (PJ014507012022) from the Rural Development Administration, Korea.
