Rapid Identification of Diaporthe citri by Gene Sequence Analysis
Article information
Abstract
Citrus melanoses caused by Diaporthe citri, has been one of the serious diseases in many citrus orchards of Jeju Island. To protect melanose in citrus farms, a fast and exact diagnosis method is necessary. In this study, diseased leaves and dieback twigs were collected from a total of 49 farms within March to April in 2022. A total of 465 fungal isolates were obtained from a total of 358 isolated plant samples. Among these fungal isolates, 40 representatives of D. citri isolates which were isolated from 22 twigs and 18 leaves on 23 farms were found based on cultural characteristics on potato dextrose agar and conidial morphology. Additionally, the molecular assay was carried out and compared with those by morphological diagnosis. All isolates were identified as D. citri by analyzing the sequences at the internal transcribed spacer (ITS) rDNA region using primers of ITS1/ITS4 or at β-tubulin using primer Btdcitri-F/R. Therefore, based on the present study, where the results of morphological identification of conidial type were consistent with DNA sequence analysis of certain gene, choosing a suitable method for a fast diagnosis of citrus melanose was suggested.
Introduction
Citrus cultivating area in Jeju has been exceeded from 10,000 ha in 1975 to 25,000 ha in 1996, but since 2009 it has been kept at about 20,000 ha because of the overproduction leading to lower prices (Ko, 2009). In 2021, amount of citrus production was 613,118 MT, area of farm household was 30,799 ha and the gross income from citrus production in South Korea was 1,027,131 million Korean Won (Report Name: Citrus Annual-USDA Foreign Agricultural Service; December 20, 2022) (Office of Agricultural Affairs, 2022).
More than 90 types of diseases have been reported in citrus fruits worldwide, and more than 35 diseases, including viral diseases, have been reported in Korean citrus fruits (Hyun et al., 2002). These diseases may cause the loss of citrus production and result in reducing income of farms. Melanose causes reduced fruit quality and marketability by damaging the surface of the fruit without affecting the pulp quality, which is much higher than the damage caused by other diseases or pests in Korea. The average diseased fruit rate by citrus melanose on Jeju Island from 2003 to 2012 was about 8.5%, and the average incidence rate of melanose disease was 13.5%, which has a little different incidence every year (Hyun et al., 2013).
Citrus melanose is caused by Diaporthe citri Wolf (anamorph: Phomopsis citri Fawc.) which causes two distinct diseases in the citrus plant. The perfect stage of the fungus causes melanoses in citrus, disease characterized by superficial fruit and foliar lesions. The other is in the imperfect stage which causes Phomopsis stem-end rot, a post-harvest disease (Whiteside et al., 1988). D. citri produces the small black lesions raised pustules on the leaves, twigs, and fruits (Timmer et al., 1988) and produces inoculums in dead twigs as a saprophyte (Mondal et al., 2007). They have three types of spores, one is (α) spores (colorless, monospores, 5–9×2–4 μm in size, directly invade the host plant) and the second is beta (β) spores (colorless, a filamentous dandelion, 20–30×0.7–1.5 μm in size, cannot directly invade the host plant because it does not germinate) (National Crop Pest Management System, South Korea, 2022). The last one is gamma (γ) spores (colorless, a multi-guttulata, fusiform to subcylindrical, 18–22×2.4–2.6 μm in size, an intermediate between α and β conidial types). These particular spore types have been used as a diagnosis tool (Chaisiri et al., 2020; Whiteside et al., 1988; Wolf, 1926).
The Diaporthe genus is frequently identified by using traditional molecular barcoding for fungal species discrimination based on nuclear ribosomal internal transcribed spacer regions (ITS) (Castlebury, 2005; Marin-Felix et al., 2019; Santos and Phillips, 2009; Santos et al., 2010). Due to the fact that the polymerase chain reaction (PCR)-based technique showed outstanding specificity and sensitivity, it may be used to effectively detect D. citri in practice (Chaisiri et al., 2022). Most of the studies including Chaisiri et al. (2020) used molecular identification method with universal primer to detect the D. citri for several purposes such as identification and phylogenetic study.
Two methods of identification for citrus melanose caused by D. citri were morphological identification of conidial type and DNA sequence analysis of certain gene such as ITS or β-tubulin. If the D. citri which was identified by the morphological method was consistent with the molecular biological analysis, the detection work for D. citri identification by two methods would be skipped by using one method. Therefore, in this study, D. citri was isolated from twigs and leaves of citrus plants in Jeju Island. The DNA sequences in the ITS or β-tubulin of D. citri, which were identified by morphological observation, were analyzed to find out whether the D. citri results of morphological identification were consistent with the molecular identification method.
Materials and Methods
Collection of samples
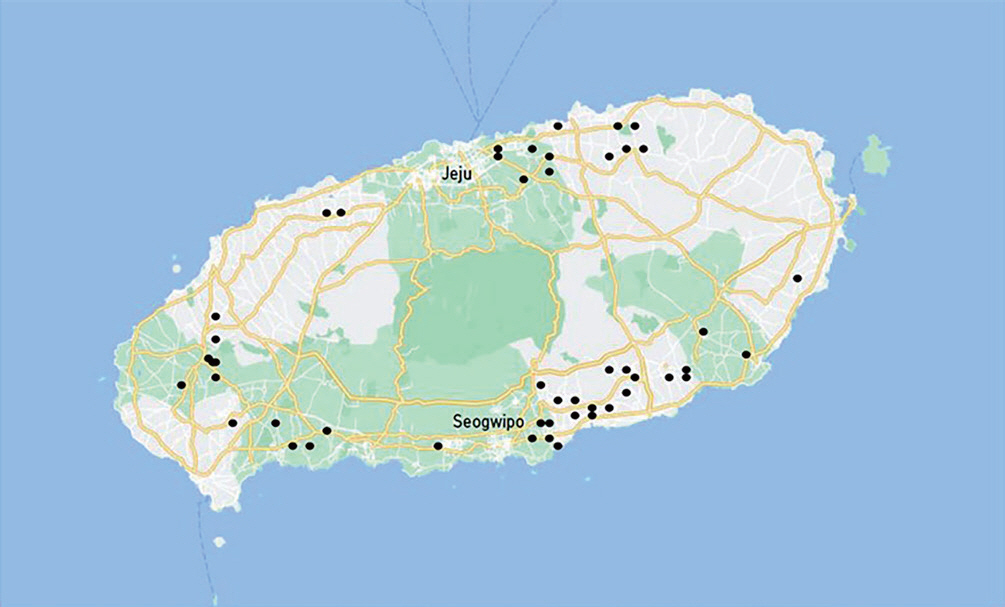
Based on characteristic symptoms, fresh specimens of diseased leaves and dieback twigs by D. citri were collected from 49 citrus orchards from four regions of the Northeastern, Southeastern, Northwestern, and Southwestern regions of Jeju Island, South Korea from March to April in 2022 (Fig. 1).
Isolation of pathogen
The D. citri was isolated from symptomatic leaves and dieback twigs showing melanose. The symptomatic tissues were cut into small pieces (5 mm×5 mm) including diseased and healthy tissues. The small pieces of plant tissues were soaked in 1% sodium hypochlorite solution (NaClO) for 30 sec, followed by 70% ethanol for 30 sec, then rinsed three times in sterile distilled water for 30 sec. The tissues were dried on sterile tissue paper and were cut as cross again on small pieces. These pieces were placed onto potato dextrose agar (PDA; Becton, Dickinson and Company, Claix, France) medium and incubated for 3–4 days at 28°C. Mycelium tips growing from the pieces of plant tissues were sub-cultured on fresh PDA medium for 7 days at 28°C.
Morphological identification
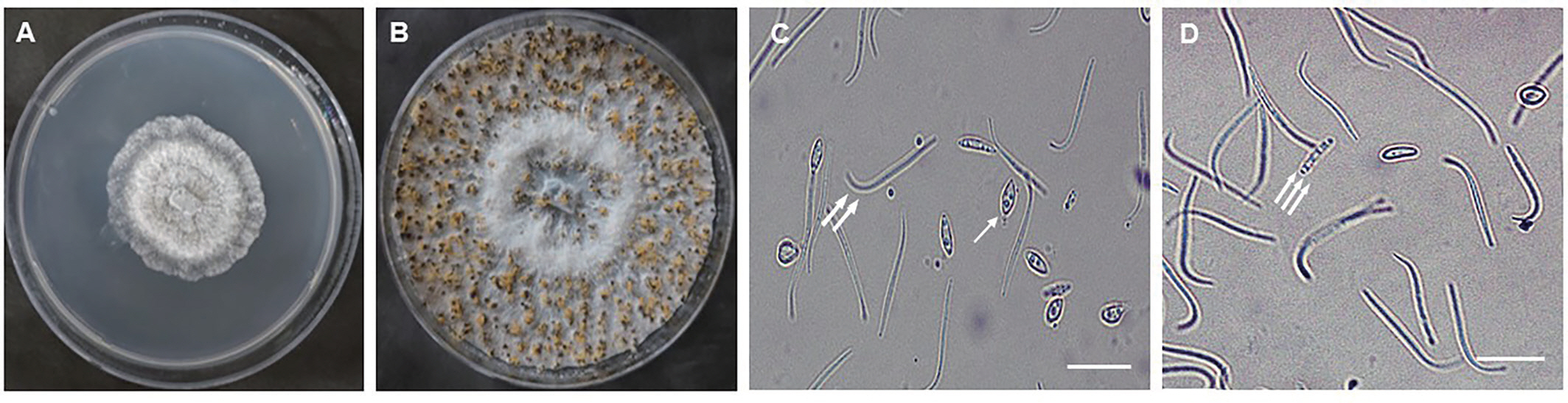
To achieve sporulation, the fungi were incubated with oatmeal agar medium (Becton, Dickinson and Company) under fluorescence light at 25°C for 4 weeks. The conidia formed on the oatmeal agar medium were observed using a light microscope (BX60, Olympus, Tokyo, Japan). The isolates containing α - and β-type form conidia (including γ-type in some cases), which are typical form of the melanose pathogen, were identified as D. citri.
Molecular identification
Representative D.citri isolates were grown in potato dextrose broth (PDB; Becton, Dickinson and Company) for genomic DNA extraction at 28°C for 7–10 days shaking at 120 rpm. Approximately 200 mg of fungal hyphae was scraped directly from PDB, and the cetyltrimethylammonium bromide method (Aamir et al., 2015) was followed with modifications. The hyphae were lyophilized in liquid nitrogen and ground with mortar and pestle. Two ml microcentrifuge tube containing ground powder and 700 μl of lysis buffer (100 mM Tris HCI [pH8.0], 50 mM EDTA, 3% sodium dodecyl sulfate) was homogenized with a WiseMix Multifuction Vortex Mixer (DAIHAN Scientific, Wonju, Korea) for 1 hr. This homogenized mixture was centrifuged at 13,000 rpm for 10 min and the supernatant was transferred to a fresh 2 ml microcentrifuge tube. Then 2 μl of RNase A (10 mg/ml) was added and the whole solution was incubated at 37°C for 30 min. Later, an equal volume of cleaning buffer (phenol:chloroform:isoamyl alcohol=25:24:1) was added, homogenized, and centrifuged at 13,000 rpm for 10 min. This step was repeated once. The upper aqueous layer was taken and transferred to a fresh 1.5 ml microcentrifuge tube. Then an equal volume of 100% ethanol was added and the DNA in this solution was precipitated at 20°C for 30 min, followed by centrifugation at 12,000 rpm for 10 min. The pellet of DNA was washed with 70% ethanol and centrifuged again at 12,000 rpm for 5 min. Finally, the DNA pellets were air-dried overnight and dissolved in 1× TE buffer. DNA products were detected in 0.7% agarose electrophoresis gel and stained with 1 μl dyne loading star to UV light visualization using a UVP trans illuminator (UV solo touch, Analytik Jena AG, Jena, Germany).
Amplification and PCR sequencing were obtained from an ITS with primer set of ITS1 (5′-TCCGTAGGTGAACC TGC-GG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al., 1990) or from β-tubulin with primer set of Btdcitri-F (5′-GACCCGACTCGACAATAACG-3′) and Btdcitri-R (5′-ATTCACTCCTCGCCCTCAAG-3′). The specific primer set of Btdcitri was designed using Clone manager (v.9.0) and Bio Edit (v.7.2.5) software targets to β-tubulin gene (NCBI genomic sequence: NW_025064848.1) from whole genome sequence of D. citri (NCBI reference genome: ASM1459564v1). Furthermore, the amplification was performed in a 20 μl reaction containing 2 μl of DNA template, 2 μl (10 pmol) of each forward and reverse primer, 2 μl of 25 mM of MgCl, 2 μl of 10 mM dNTPs mix, 2 μl of 10× PCR buffer, 0.5 μl (5 U/μl) of Taq DNA polymerase (iNtRON Biotechnology Inc., Seoul, Korea) and 9.5 μl of distilled water. Also, PCR was performed using a thermal cycler (Takara TP6000 Gradient, Takara Bio Inc., Tokyo, Japan). The parameters for PCR of ITS region were initial preheat at 95°C for 3 min, followed by 30 cycles of denaturation at 95°C held for 30 sec, annealing for 50 sec, at 55°C and extension at 72°C for 2 min, with a final extension stage at 72°C held for 5 min. For β-tubulin the initial preheat was at 94°C for 2 min, followed by 30 cycles of denaturation at 94°C held for 30 sec, annealing for 30 sec, at 58.2°C and extension at 72°C for 2 min, with a final extension stage at 72°C held for 4 min. PCR products were also detected with the same method as DNA. The PCR products were purified with purification kit (PCR purification kit, Genesgen, Busan, Korea). Then nucleotide sequences of the products were analyzed at Macrogen (Seoul, Korea) with primers ITS and Btdcitri. The Bio Edit software was used to align the forward and reverse sequences obtained from the Macrogen results. Then the aligned sequences were compared with sequences in the GenBank database using the NCBI BLAST program (http://blast.ncbi.nim.nih.gov/Blast.cgi).
Results
Isolation of D. citri
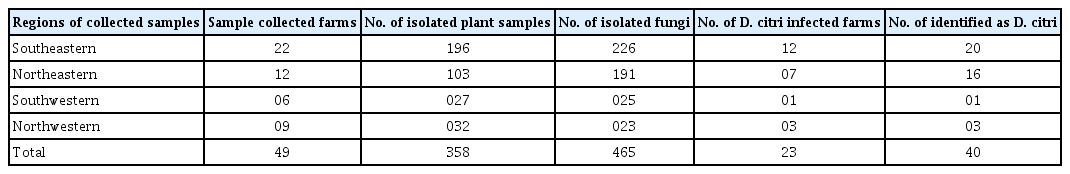
A total of 465 fungal isolates were obtained from a total of 358 isolated plant samples, which were collected from a total of 49 farms: 22 in the Southeastern region, 12 in the Northeastern region, 6 in the Southwestern region, and 9 in the Northwestern region (Table 1, Fig. 1). Among these fungal isolates, 40 representative isolates were selected as candidates of D. citri based on cultural characteristics of mycelium on PDA.
Morphological identification
The mycelium of the selected isolates was fan-shaped, white, and flat or effuse alternate to low convex. The conidial mass was initially hyaline to yellowish, becoming white to cream conidial droplets after 25–30 days in light at 25°C. Finally, two types of conidia were observed under light microscope, one is the α-type, which was hyaline, unicellular, and fusiform to ellipsoidal. The other is β-type, which was hyaline, unicellular, filiform, curved, and often strongly hooked (Fig. 2). The 40 selected isolates were identified as D. citri based on the observation of α - and β-type conidia.
Molecular identification
The molecular identification of the 40 representative isolates chosen based on the conidial morphology revealed that they were D. citri, Diaporthe sp., and Phomopsis sp. stains. The total DNA of the 40 strains in a 0.7% agarose gel was shown (Fig. 3). The ITS gene was amplified in size of 604 bp (Fig. 4) and β-tubulin was in 1,967 bp (date not shown). The blast search at NCBI indicated that all isolates held between 94.6% and 100% of the D. citri, Diaporthe sp., and Phomopsis sp. stains after sequencing by Macrogen and aligning by BioEdit software (Table 2) where the higher identity was chosen between ITS and β-tubulin analysis.
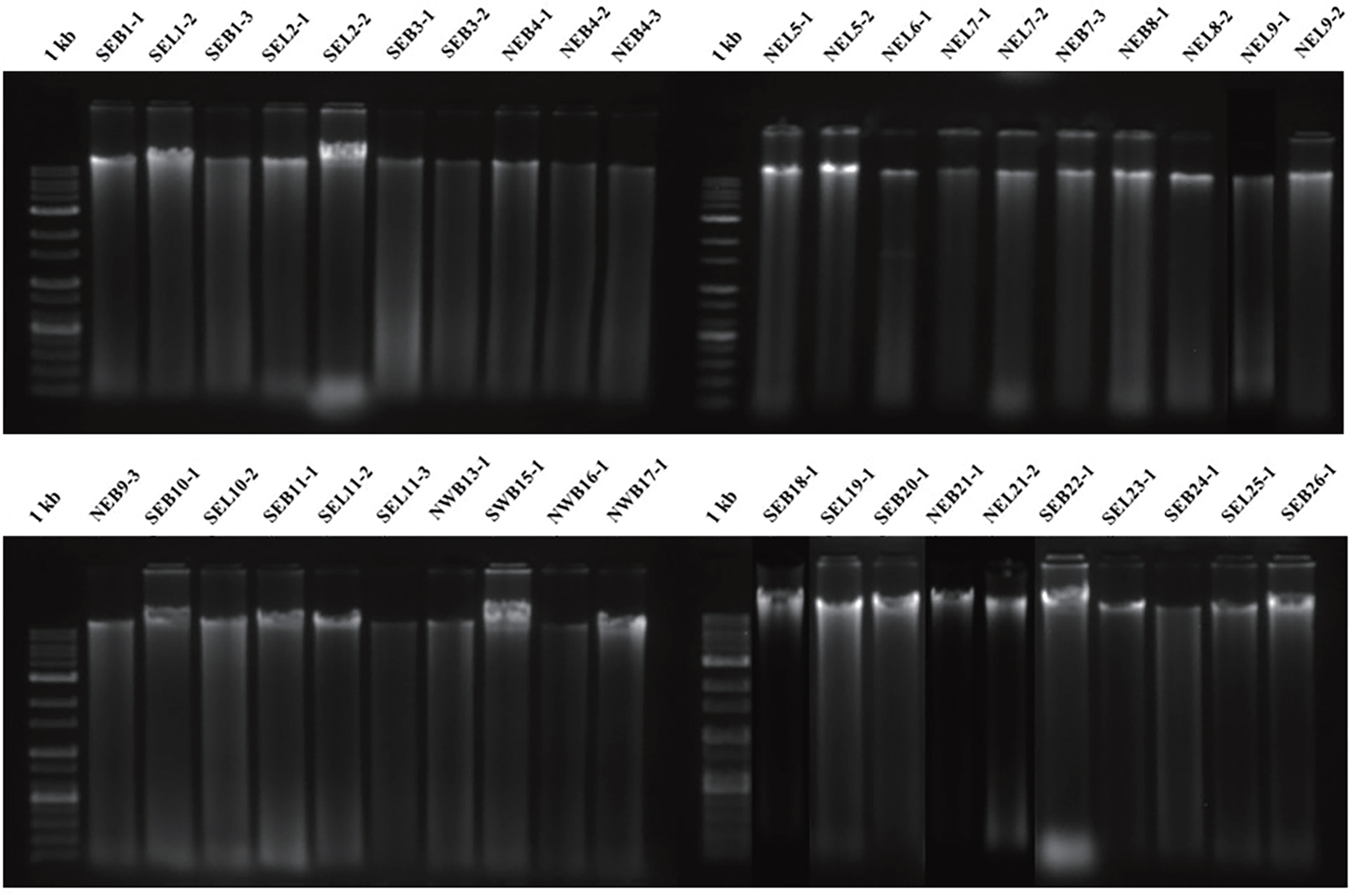
Gel electrophoresis results of total DNA of forty isolates identified as Diaporthe citri by morphological diagnosis. 1 kb, ladder; SEB, “Southeastern Branch”; SEL, “Southeastern Leaf”; NEB, “Northeastern Branch”; NEL, “Northeastern Leaf”; SWB, “Southwestern Branch”; NWB, “Northwestern Branch”. The number indicates farm-sample serial number.
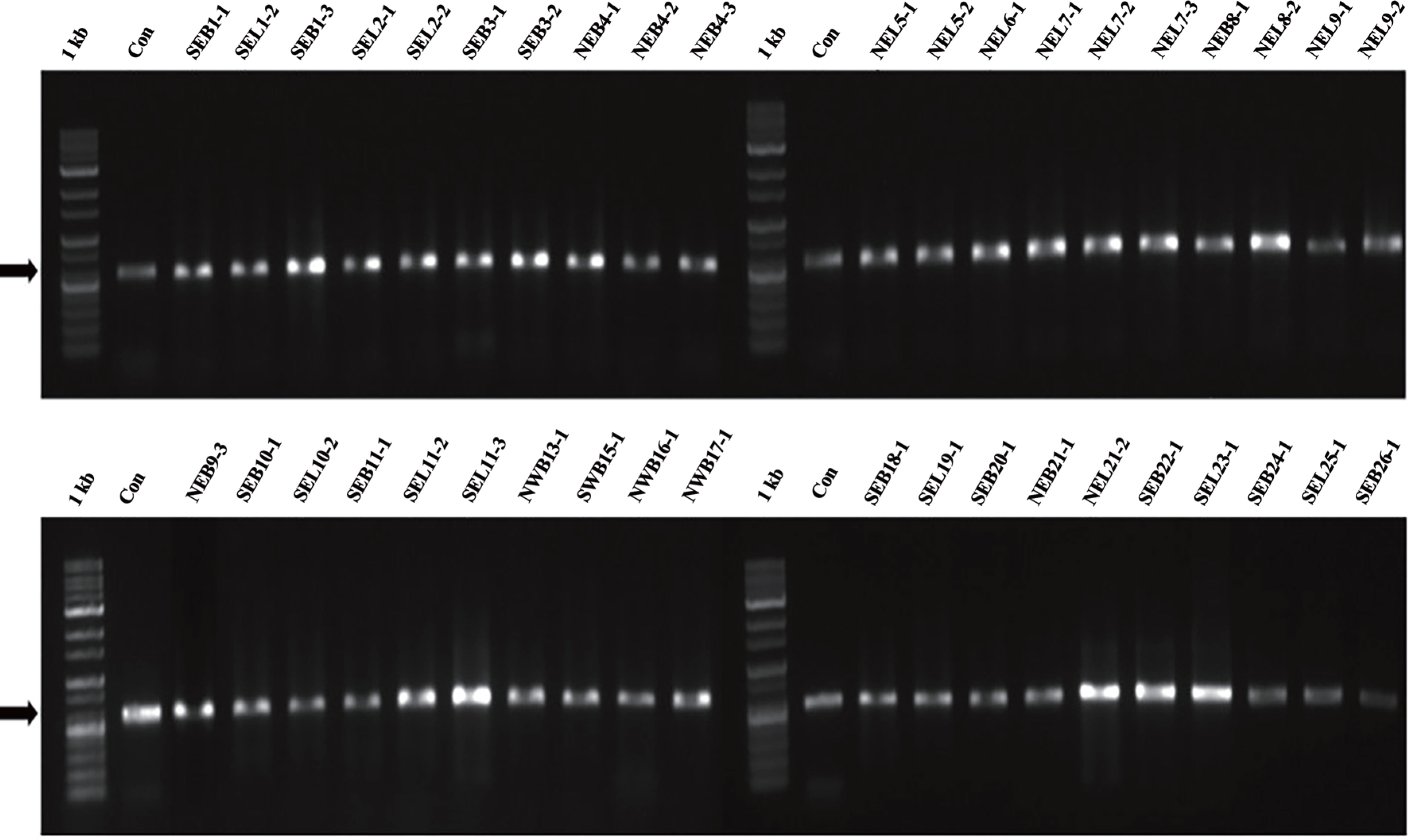
Molecular identification of the pathogen causing citrus melanose by Diaporthe citri. Gel electrophoresis of polymerase chain reaction-amplified internal transcript spacer (ITS) regions from the 40 isolates identified as D. citri by morphological diagnosis using the universal primers ITS1/ITS4. 1 kb, ladder; Con, D. citri 6 from the Plant Pathology Laboratory of Jeju National University; SEB, “Southeastern Branch”; SEL, “Southeastern Leaf”; NEB, “Northeastern Branch”; NEL, “Northeastern Leaf”; SWB, “Southwestern Branch”; NWB, “Northwestern Branch”. The number indicates farm-sample serial number. Arrows indicate 604 bp.
Discussion
Forty D. citri isolates, which were selected based on conidial morphology were in a line with the studies of Kwon et al. (2003), Gopal et al. (2014), and Chaisiri et al. (2020). The fan-shaped and white mycelium of D. citri on PDA in this study coincided with that of Timmer et al. (2004) study in which mycelium was typically fan-shaped and white in color. Moreover, the morphology of conidia was also similar to the study of Chaisiri et al. (2020) whereas conidial mass was initially hyaline to yellowish, becoming white to cream conidial droplets exuding from central ostioles after 25 days in light at 25°C. Finding α-, β-, and γ-types of D. citri conidia and its size, shape, and color has been reported in a number of studies (Chaisiri et al., 2020; Gopal et al., 2014; Kwon et al., 2003) which was observed also in this study (Fig. 2).
After confirmation of 40 representative D. citri isolates with morphological characteristics, the molecular identification with PCR assay also revealed that these were held between 94.6% and 100% of the D. citri, Diaporthe sp., and Phomopsis sp. stains by NCBI blast analysis. Like the present study, the identification based on ITS region sequence and β-tubulin sequence analysis showed that 38 representative isolates, which were selected based on morphological characteristics, from totally of 140 isolates of different tissues of citrus, i.e., leaves, fruits, and twigs belong to Diaporthe species (Chaisiri et al., 2020).
Since the past two decades, molecular identification through DNA barcoding of fungi has become an integrated and essential part of fungal ecology research which has provided new insights into the diversity and ecology of many different groups of fungi (Anderson and Cairney, 2004; Chase and Fay, 2009; Horton and Bruns, 2001; Seiffert, 2009). Studies by Freeman et al. (2009), Frohlich-Nowoisky et al. (2009), Lindahl et al. (2007), O'Brien et al. (2005), Pickles et al. (2010), and Zinger et al. (2009) have revealed that sequence-based analysis of environmental samples (‘environmental barcoding’) allowed the abundance and species richness of fungi to be determined at a high rate and more reliably than conventional biotic surveys. For the identification of single taxa and mixed environmental templates (“environmental DNA barcoding”), the ITS of nuclear DNA is the preferred DNA barcoding marker.
In this study, it was revealed that all fungal isolates producing α-, β-, and γ-types conidia were identified as D. citri by DNA barcoding. These results suggested that in the case of diagnosis for citrus melanose alternative methods, either morphological identification or molecular biological analysis may be possible in order to fast diagnosis.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
This work was carried out with the support of ‘Cooperative Research Program for Agriculture Science and Technology Development (PJ0169062023)’ funded by Rural Development Administration, Republic of Korea.