Introduction
Materials and Methods
Greenhouse experiment
Measurement of growth and disease incidences in tomato plants
Total bacterial and fungal populations in soil
Enzyme activity in soil
Statistical analysis
Results
Growth and yield parameters of tomato plants
Table 1
DAT, days after transplanting; Chemical fertilizer (F), bacterial grass culture (G), 1/3 volume of G plus 2/3 F (GF), and F plus synthetic fungicide (active ingredients 45% carbendazim 45% and 95 metalaxyl-M, Lidochamgold, Seongbo Chemical) (FSf). The experiments to investigate the effect of a bacterial grass culture on tomato growth and fungal disease control were carried out in the greenhouses of Chonnam National University in Gwangju, Korea. Soil type at this site is a loam (http://soil.rda.go.kr/geoweb). The tomato cultivar used was Younghwa (Daenong Company). Tomato seeds were sown in bed soil (Bio bed soil I, Heong Nong Seed, Korea) in 54×45 mm2 plastic cell plug trays, and then grown at 24°C and 60% relative humidity in an artificially illuminated room (12,000 lux) with a 16-h photoperiod. Twelve four-week-old seedlings were transplanted in a plot with a raised bed with a row spacing of 0.6 m and a plant spacing of 0.4 m within rows on August 21 of 2013. The experimental area of one plot was a 2.5×1.0 m2. The experiment was arranged in a randomized complete block design (RCBD) with four treatments and three replications. Treatments were G, F, GF and FSf. To prepare G for use in this experiment, a grass culture medium (g/l; fresh Kentucky blue grass powder 178; urea 2.8; single superphosphate 8.8; potassium chloride 1.6; magnesium sulfate 1.0; calcium chloride 1.0; yeast 0.1; sodium molybdate 0.05; zinc sulfate 0.06; crab shell powder (Purne Co., Korea) 0.8; gelatin powder (Geltech Co., Korea) 0.2) was autoclaved, inoculated with P. ehimensis KWN38 and incubated for 5 days at 30°C. The F treatment (10.2: 5.15: 6.1 kg; N: P2O5: K2O per 1,000 m2) was applied directly to soil using urea for N, mono superphosphate for P2O5, and potassium chloride for K2O by the chemical fertilizer recommendation for greenhouse tomatoes in Korea on July 21, September 21, and October 21 of 2013. A half amount of the total chemical fertilizer for each F, FSf, and GF treatment was applied to the soil by side-dressing as a basal application before transplanting the seedlings, while the remaining half was divided into two more side dressings applied one and two months after transplanting the seedlings. Chemical fertilizer was dissolved with distilled water and used. The GF treatment received 1/3 volume of G with 2/3 amount of F. Ninety-seven ml and 32 ml of G were treated weekly to the root zone of each tomato plant in the G and GF, respectively, treatments for 16 weeks. After the FSf treatment was prepared by dissolving 1.0 g fungicide into 1 l of distilled water, it was applied at the same time in each plot. Three additional sprays in all treatments were applied to the foliar portion of the plants every 20 days between 60 days after transplanting and 90 DAT after the first symptoms of early blight and gray mold disease occurred on the leaves and stems. For foliar application, a half amount of the total chemical fertilizer for F was applied after dissolving with distilled water. Ninety-seven ml and 32 ml of G were applied to the leaf surface of one tomato plant in the G and GF, respectively. The results did not indicate a cumulative value, but average per plant. LSD, Least significant difference. Means with a common letter in the same column and sampling time are not significantly different each other at the 0.05 level.
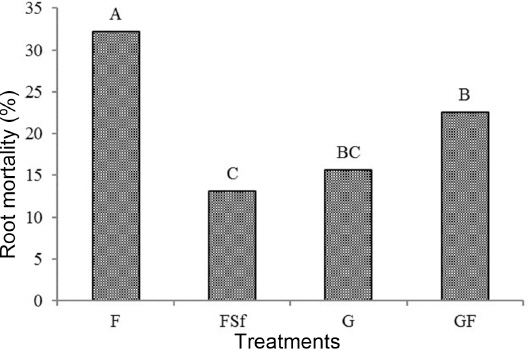
Root mortality
Fig. 1
Fungal disease incidence on leaves and stems of tomato plants
Fig. 2

Table 2
DAT, days after transplanting; Chemical fertilizer (F), bacterial grass culture (G), 1/3 volume of G plus 2/3 F (GF), and F plus synthetic fungicide (active ingredients 45% carbendazim 45% and 95 metalaxyl-M, Lidochamgold, Seongbo Chemical) (FSf). The experiments to investigate the effect of a bacterial grass culture on tomato growth and fungal disease control were carried out in the greenhouses of Chonnam National University in Gwangju, Korea. Soil type at this site is a loam (http://soil.rda.go.kr/geoweb). The tomato cultivar used was Younghwa (Daenong Company). Tomato seeds were sown in bed soil (Bio bed soil I, Heong Nong Seed, Korea) in 54×45 mm2 plastic cell plug trays, and then grown at 24°C and 60% relative humidity in an artificially illuminated room (12,000 lux) with a 16-h photoperiod. Twelve four-week-old seedlings were transplanted in a plot with a raised bed with a row spacing of 0.6 m and a plant spacing of 0.4 m within rows on August 21 of 2013. The experimental area of one plot was a 2.5×1.0 m2. The experiment was arranged in a randomized complete block design (RCBD) with four treatments and three replications. Treatments were G, F, GF and FSf. To prepare G for use in this experiment, a grass culture medium (g/l; fresh Kentucky blue grass powder 178; urea 2.8; single superphosphate 8.8; potassium chloride 1.6; magnesium sulfate 1.0; calcium chloride 1.0; yeast 0.1; sodium molybdate 0.05; zinc sulfate 0.06; crab shell powder (Purne Co., Korea) 0.8; gelatin powder (Geltech Co., Korea) 0.2) was autoclaved, inoculated with P. ehimensis KWN38 and incubated for 5 days at 30°C. The F treatment (10.2: 5.15: 6.1 kg; N: P2O5: K2O per 1,000 m2) was applied directly to soil using urea for N, mono superphosphate for P2O5, and potassium chloride for K2O by the chemical fertilizer recommendation for greenhouse tomatoes in Korea on July 21, September 21, and October 21 of 2013. A half amount of the total chemical fertilizer for each F, FSf, and GF treatment was applied to the soil by side-dressing as a basal application before transplanting the seedlings, while the remaining half was divided into two more side dressings applied one and two months after transplanting the seedlings. Chemical fertilizer was dissolved with distilled water and used. The GF treatment received 1/3 volume of G with 2/3 amount of F. Ninety-seven ml and 32 ml of G were treated weekly to the root zone of each tomato plant in the G and GF, respectively, treatments for 16 weeks. After the FSf treatment was prepared by dissolving 1.0 g fungicide into 1 l of distilled water, it was applied at the same time in each plot. Three additional sprays in all treatments were applied to the foliar portion of the plants every 20 days between 60 days after transplanting and 90 DAT after the first symptoms of early blight and gray mold disease occurred on the leaves and stems. For foliar application, a half amount of the total chemical fertilizer for F was applied after dissolving with distilled water. Ninety-seven ml and 32 ml of G were applied to the leaf surface of one tomato plant in the G and GF, respectively. The level of fungal disease incidence is calculated as the ratio of diseased leaf number per total leaf number. LSD, Least significant difference. Means with a common letter in the same column and sampling time are not significantly different each other at the 0.05 level.
Correlations between disease incidence and growth parameters
Table 3
| Diseased leaf | Diseased stem | Shoot length | Shoot fresh weight | Shoot dry weight | Root fresh weight | Root dry weight | Fruit number | Fruit weight | |
|---|---|---|---|---|---|---|---|---|---|
| Diseased leaf | 1.000 | ||||||||
| Diseased stem | -0.700 | 1.000 | |||||||
| Shoot length | 0.723 | -0.999* | 1.000 | ||||||
| Shoot fresh weight | 1.000** | -0.700 | 0.723 | 1.000 | |||||
| Shoot dry weight | 0.966 | -0.491 | 0.519 | 0.966 | 1.000 | ||||
| Root fresh weight | 0.933 | -0.397 | 0.427 | 0.933 | 0.994 | 1.000 | |||
| Root dry weight | 0.913 | -0.349 | 0.379 | 0.913 | 0.988 | 0.999* | 1.000 | ||
| Fruit number | 0.979 | -0.541 | 0.569 | 0.979 | 0.998* | 0.987 | 0.977 | 1.000 | |
| Fruit weight | 0.984 | -0.564 | 0.590 | 0.984 | 0.996 | 0.982 | 0.971 | 0.999* | 1.000 |
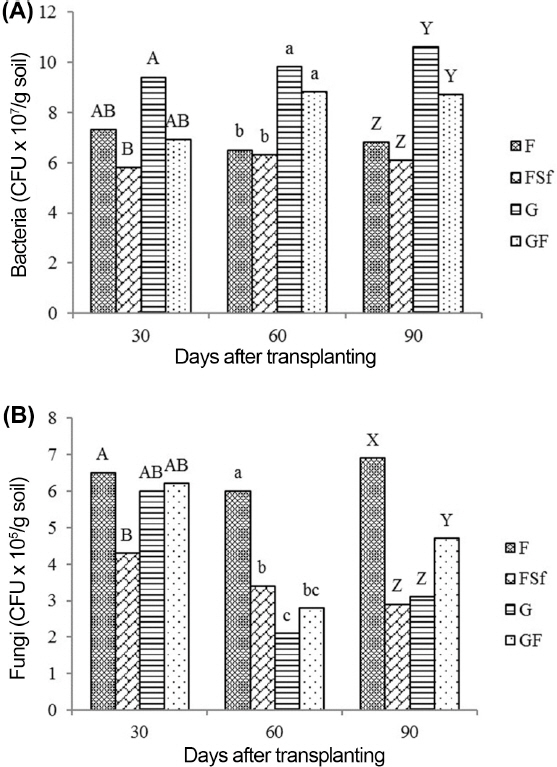
Bacterial and fungal populations in the soil
Fig. 3
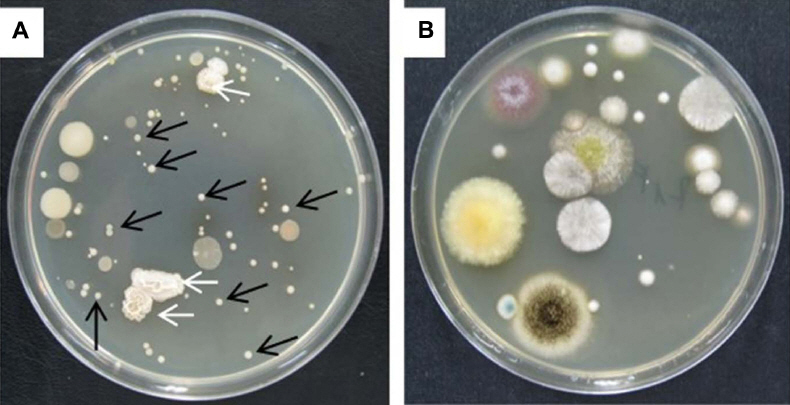
Fig. 4
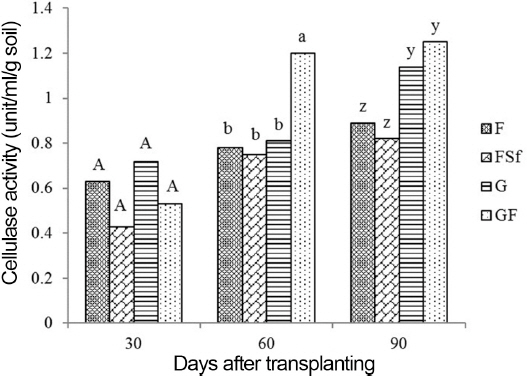
Enzyme activity in soil
Fig. 5
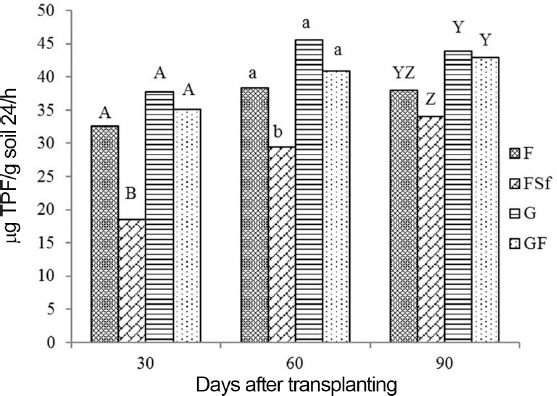
Fig. 6



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print