Survey and Screening of Fungicide for the Control of Tomato Black Leaf Mold Pseudocercospora fuligena
Article information
Abstract
Tomato black leaf molds were collected from the six metropolitan cities, which were occurred mainly from the end of August until November. There was no significant difference on the fungal growth between potato dextrose agar and tomato-oatmeal agar media. The mycelial growth of the fungus was robust at a relatively high temperature, from 28 to 30°C. The suppression rates of hyphal growth ranged from 17-98% on the media supplemented with four different chemicals such as difenoconazole, fluquinconazole and prochloraz manganese complex, metconazole, and flutianil and there is no different suppression rates of the fungicides on the tested Pseudocercospora fuligena isolates.
In September 2011, tomato black leaf mold (Pseudocercospora fuligena) was reported for the first time in Korea on tomato cultivation farms in Chungso-myeon, Poryung City, Choongnamdo Province, and Kyuam-myeon, Buyeo-gun, Choongnamdo Province (Lee et al., 2012). The first instance of the disease was reported in the Philippines in 1936 (Roldan, 1938). Tomato black leaf mold was known to occur in tropical and sub-tropical regions featuring hot and humid weather (Wang et al., 1997). The affected areas have been reported to include Asia (Blazque, 1991; Chandrasrikul, 1962; Govindu and Thirumalachar, 1954; Mohanty and Mohanty, 1955; Mulder and Holliday, 1975; Roldan, 1938; Turner, 1971; Wang et al., 1997; Yamada, 1951), Africa (Mulder and Holliday, 1975), Oceania (Johnston, 1963), North America (Blazque and Alfieri, 1974; Johnston, 1963) and Brazil (Halfeld-Vieira et al., 2006).
The Southeast Asian region is suffering noticeable damages by the tomato black leaf mold because of the favorable temperature (28 ± 2°C) and relative humidity (71-99%) for development of the disease (Hartman et al., 1991; Hartman and Wang, 1992; Roldan, 1938; Wang, 1997). The climate of Korea is gradually moving toward a sub-tropical type, which may cause the disease to spread wider in the country. The black leaf mold of tomato was known to cause an approximate 32% reduction in yield (Hartman and Wang, 1992) with 68% loss of leaves (Mersha and Hau, 2009). In addition to tomato, other plants of the solanaceae family such as pepper and eggplant were vulnerable by this fungus (Wang et al., 1997). Thus, it is necessary to investigate the incidence of the disease in other plant species too.
In this study, we investigated (a) the occurrence of the disease by tomato black leaf mold to compare the previous results reported in 2011, and (b) the cultivation traits to prevent spreading of the disease and the suppression rate of the hyphal growth depending on chemical treatments.
The results of survey conducted in six metropolitan cities of between 2012 and 2013 indicated that black leaf mold occurs in all the surveyed areas. The disease occurred independently 36 and 60% of the time along with other diseases such as mycosphaerella leaf spots (Table 1 and Fig. 1). This is most likely because leaf mold or mycosphaerella leaf spots occur in a humid environment just as the black leaf mold does. The plants affected by the disease included all the variations in the study area, suggesting that no resistant varieties are found domestically (Table 1). It was found that black leaf mold occurred mostly from the end of August to the end of October (Table 1). The occurrence of disease is facilitated by frosting and that is why it occurs during the rainy season in Taiwan (Hartman and Wang, 1992). Conidia did not germinate at or below 84.5% relative humidity (Hartman et al., 1991). It was thought that the disease occurs in the autumn because frosting occurs due to difference in temperature between the daytime and the nighttime, and high relative humidity in the greenhouse while doors are kept closed during the night.
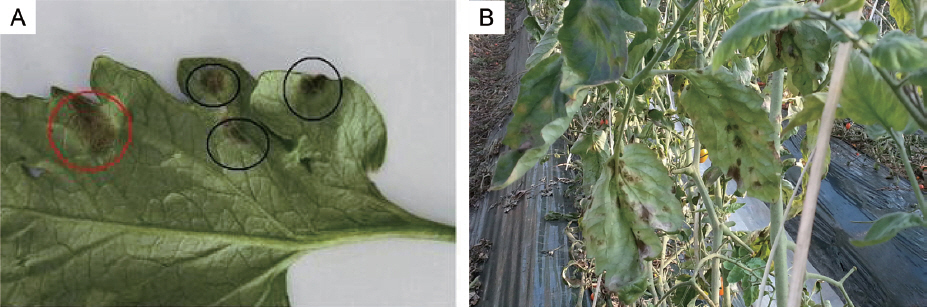
Black leaf mold and Leaf mold growing on tomato leaf. (A) Black circle indicates black leaf mold, and red circle indicates leaf mold. (B) Symptoms of black leaf mold on tomato.
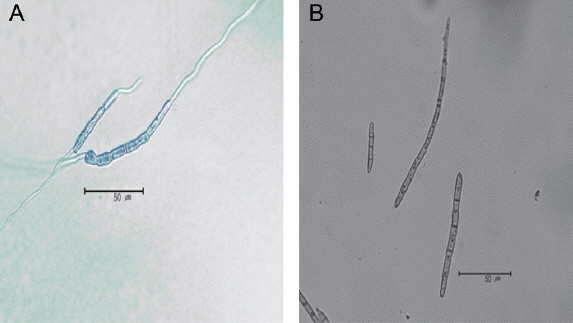
Black leaf mold is particularly difficult to isolate and to cultivate because of slow growth. In this study, single spore was isolated on water agar containing 0.4% streptomycin, and then placed into a potato dextrose agar (PDA) medium and incubated at 28°C. After 7 days, the diameter of the fungal colony was 2 mm (Fig. 2C). When the 8 mm hypha disc was placed on PDA medium, colony diameter reached to 35 mm after 30 days of incubation (Table 2, Fig. 2A and B). To explore the most suitable medium and temperature for black leaf mold, PDA, tomatooatmeal agar (TOA) (tomato leaf 50 g, oatmeal 15 g, CaCO3 1 g, agar 20 g, and distilled water 1 l) (Hartman and Wang, 1991) and V-8 juice agar (V-8 juice 175 ml, CaCO3 3 g, agar 20 g, and distilled water 1 l) were used as culture media, and three different temperatures (25, 28 and 30°C) were tested. The fungi grew well on TOA and PDA media compared to V-8 medium (Table 2). This result is consistent with the previous observations by Mersha (2008). The growth rate was faster at 28 and 30°C compared to that of 25°C (Table 2). These results are similar to those previously reported (Hartman et al., 1991; Mersha, 2008). In addition, we observed needle-shaped conidia having several septations (Fig. 3).

Fungal growth of Pseudocercospora fuligena on PDA. (A and B) Photos were taken 30 days after inoculation of 8 mm of culture agar block. A, top of plate; B, bottom of plate (C) Black leaf mold grown from single spore on PDA. A photo was taken 7 days after inoculation.
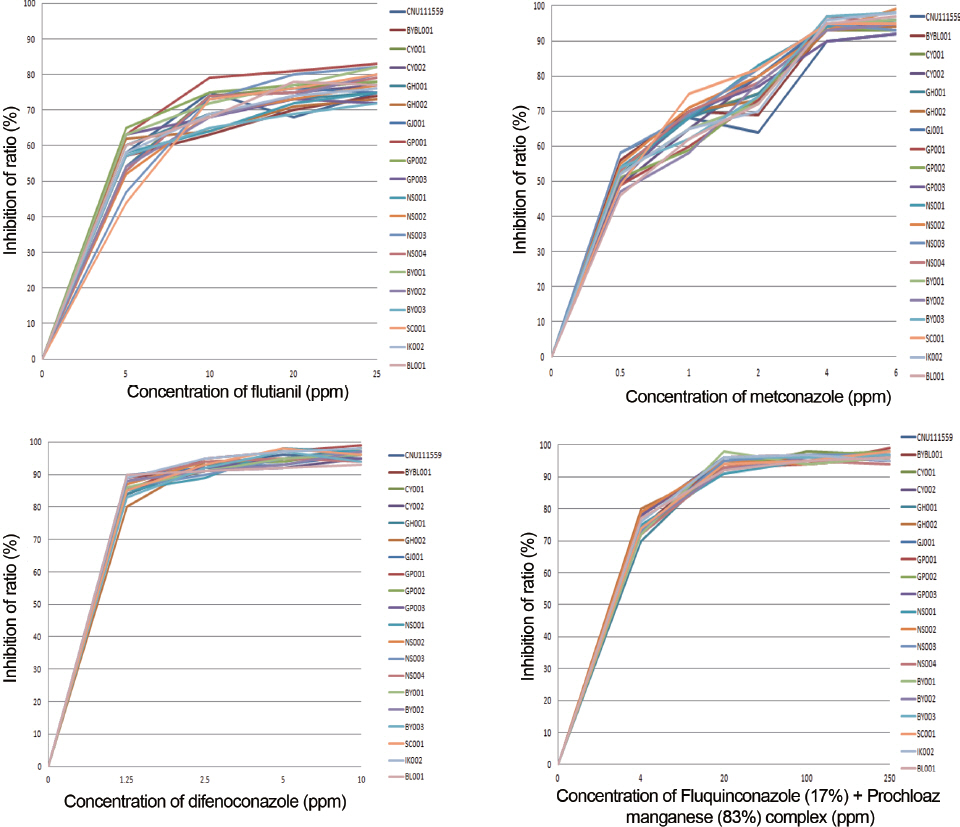
To date, no chemicals have been registered Korea for the control of black leaf mold. Thus, prompt identification and registration of chemicals are urgently needed. In this study, spore growth suppression rates of four chemicals were measured at several different concentrations; 5, 10, 20 and 25 μg/ml of flutianil, 0.5, 1, 2, 4 and 6 μg/ml of metconazole, 1.25, 2.5, 5 and 10 μg/ml of diefconazole, 0, 4, 20, 100 and 250 μg/ml of fluquin-conazole (17%) + prochloraz manganese complex (83%).
Eight-millimeter hyphal discs from 20 P. fuligena strains collected in 2011 and 2012 were put on media, and hypha growth suppression rates were measured after 40 days. The growth rates were reduced upto 64-78% and 68-80% by flutianil with the concentrations of 10 and 25 μg/ml, respectively, and there were no differences over 10 μg/ml. Metconazole showed suppressive effects on fungal growth rate (48-59% and 90-97%) at concentrations of 0.5 and 4 μg/ml, respectively. On the chemical culture medium containing difenoconazole, the hypha growth suppression rates were 81-90% and 92-98% at concentrations of 1.25 and 2.5 μg/ml, respectively and no significant difference was observed with the increment in concentration. On the chemical culture medium of fluquinconazole (17%) and prochloraz manganese complex (83%), the growth suppression rates were 70-71% at 4 μg/ml and 91-98% at 20 μg/ml. All of the 20 strains used for this assay showed similar suppression rates of hyphal growth in each of the chemical culture media, suggesting that there are no chemical-resistant strains (Fig. 4).
Acknowledgements
This research was supported by Advanced Production Technology Development Program, Ministry of Agriculture, Food and Rural Affairs.