Carling, D. E. 1996. Grouping in
Rhizoctonia solani by hyphal anastomosis reaction. In:
Rhizoctonia Species Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control, eds. by B. Sneh, S. Jabaji-Hare, S. Neate and G. Dijst, pp. 37-47. Kluwer Academic Publishers, Dordrecht, The Netherlands.

Carling, D. E., Baird, R. E., Gitaitis, R. D., Brainard, K. A. and Kuninaga, S. 2002. Characterization of AG-13, a newly reported anastomosis group of
Rhizoctonia solani.
Phytopathology 92: 893-899.


Kim, W. G. 1996. Pathogenicity of anastomosis groups and cultural types of Rhizoctonia solani isolates on crops. Korean J. Plant Pathol. 12: 21-32.
Kim, W.-G., Cho, W.-D. and Lee, Y.-H. 1994. Anastomosis groups and cultural characteristics of Rhizoctonia solani isolates from crops in Korea. Korean J. Mycol. 22: 309-324.
Kim, W. G., Cho, W. D. and Ryu, H. Y. 1995. Diagnosis and Control of Rhizoctonia Diseases on Crops. National Agricultural Science and Technology Institute, Suwon, Korea. pp. 167.(In Korean)
Kim, W.-G., Lee, G.-B., Shim, H.-S. and Cho, W.-D. 2022. Stem rot of bonnet bellflower caused by Rhizoctonia solani AG-4. Korean J. Mycol. 50: 61-64.
Kim, W.-G., Shim, H.-S., Lee, G.-B. and Cho, W.-D. 2020. Damping-off of edible amaranth caused by Rhizoctonia solani AG-4. Korean J. Mycol. 48: 325-328.
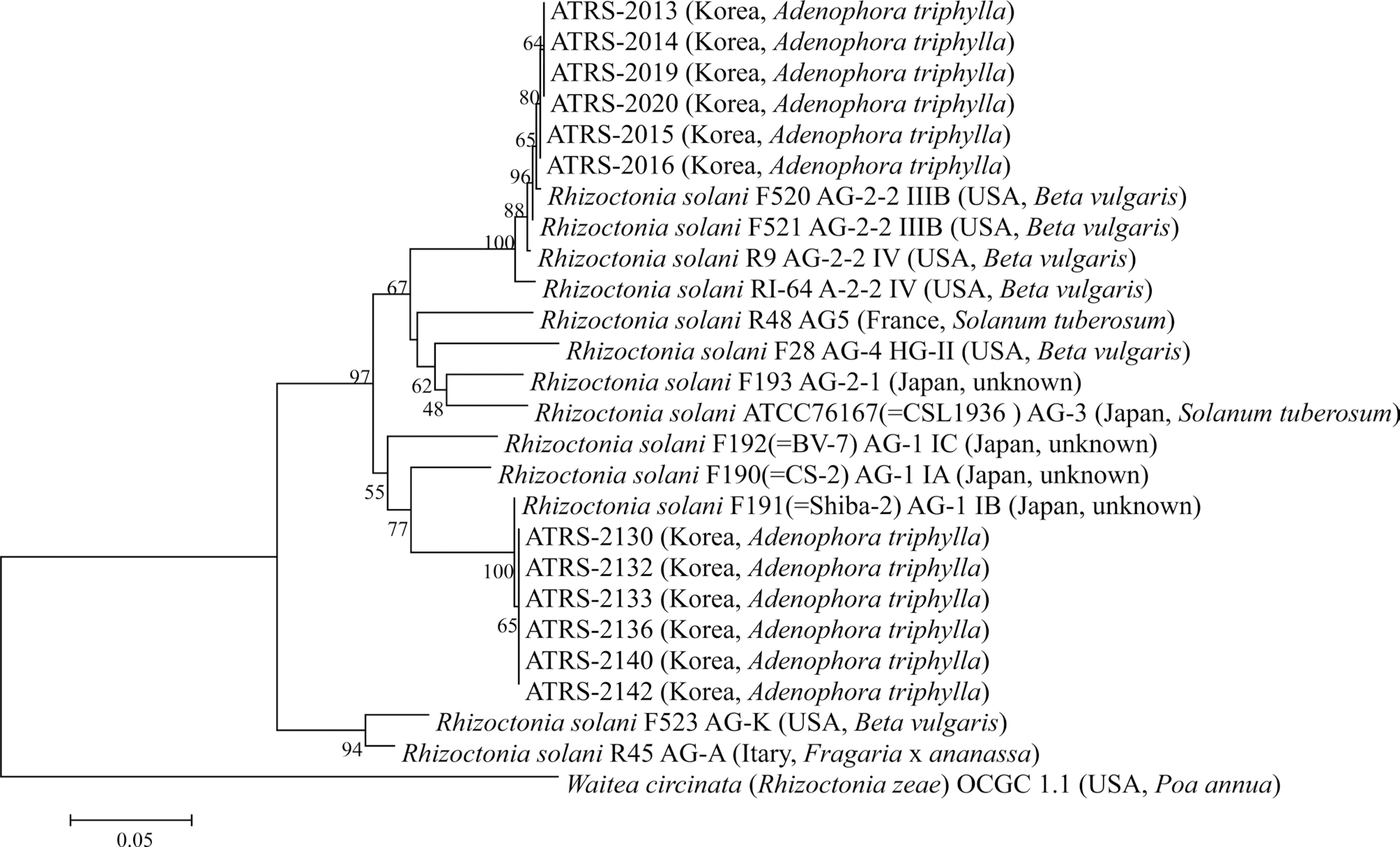
Kumar, S., Stecher, G., Li, M., Knyaz, C. and Tamura, K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms.
Mol. Biol. Evol. 35: 1547-1549.



Kuninaga, S. 2002. Current situation of the taxonomy of the genus Rhizoctonia and the R. solani species complex. Jpn. J. Phytopathol. 68: 3-20. (In Japanese)
Parmeter, J. R. Jr. and Whitney, H. S. 1970. Taxonomy and nomenclature of the imperfect state. In:
Rhizoctonia solani: Biology and Pathology, ed. by J. R. Parmeter, pp. 7-19. University of California Press, Berkeley, CA, USA.

Plants of the World Online. 2023.
Adenophora triphylla (Thunb.) A.DC. Royal Botanic Garden, Kew. URL
https://powo.science.kew.org/ [31 July 2023].
Sneh, B., Burpee, L. and Ogoshi, A. 1991. Identification of Rhizoctonia species. APS Press, St. Paul, MN, USA. pp. 133 pp.
Watanabe, B. and Matsuda, A. 1966. Studies on the grouping of Rhizoctonia solani Kühn pathogenic to upland crops. Appointed Experiment (Plant diseases and insect pests). Bulletin No. 7. Agric. For. Fish. Res. Counc. and Ibaraki Agric. Exp. Stn. Jpn. 7: 1-131. (In Japanese)
White, T. J., Bruns, T., Lee, S. and Taylor, J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a Guide to Methods and Applications, eds. by M. A. Innis, D. H. Gelfand, J. J. Sninsky and T. J. White, pp. 315-322. Academic Press, San Diego, CA, USA.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print