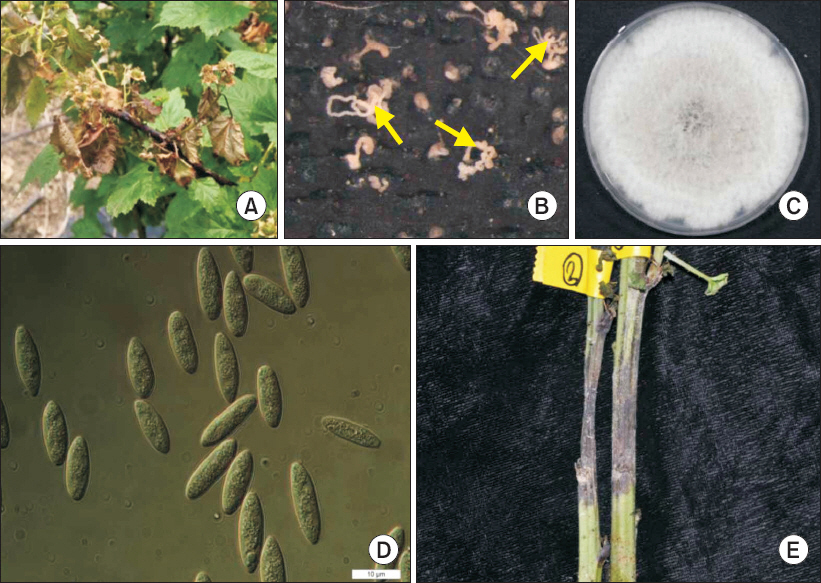
Abdollahzadeh, J, Zare, R and Phillips, A. J Phylogeny and taxonomy of
Botryosphaeria and
Neofusicoccum species in Iran, with description of
Botryosphaeria scharifii sp. nov.
Mycologia 2013. 105: 210-220.


Carbone, I and Kohn, L. M A method for designing primer sets for the speciation studies in filamentous ascomycetes.
Mycologia 1999. 91: 553-556.

Cheon, W, Kim, Y. S, Lee, S. G, Jeon, Y. H and Chun, I. J First report of branch dieback of walnut caused by
Neofusicoccum parvum in Korea.
Plant Dis 2013. 97: 1114

Choi, I. Y, Sharma, P. K and Cheong, S. S First report of
Neofusicoccum parvum associated with bark dieback of blueberry in Korea.
Plant Pathol. J 2012. 28: 217

Haleem, R. A, Abdullah, S. K and Jubrael, J. M. S Identification and pathogenicity of
Botryosphaeria parva associated with grapevine decline in Kurdistan region: Iraq.
Acta Agrobot 2012. 65: 71-78.


Korea Forest Service. 2014 Production of forest products. 2015. Daejeon, Korea. Korea Forest Service, pp. 15.(In Korean)
Kim, W. G, Koo, H. M, Kim, K. H, Hyun, I. H, Hong, S. K and Cha, J. S List of Plant Diseases in Korea. 2009. 5th ed Suwon, Korea. Korean Society of Plant Pathology, pp. 500-501. (In Korean)
Linaldeddu, B. T, Franceschini, A, Luque, J and Philips, A. J. L First report of canker disease caused by
Botryosphaeria parva on cork oak trees in Italy.
Plant Dis 2007. 91: 324

Moon, K. M, Kim, J. E, Kim, H. Y, Lee, J. S, Son, G. A, Nam, S. W, Kim, B. W and Lee, J. H Antioxidant activity of
Rubus crataegifolius Bge. fruit extracts.
J. Life Sci 2011. 21: 1214-1218.

Ni, W, Zhang, X, Bi, H, Iteku, J, Ji, L, Sun, C, Fang, J, Tai, G, Zhou, Y and Zhao, J Preparation of a glucan from the roots of
Rubus crataegifolius Bge. and its immunological activity.
Carbohydr. Res 2009. 344: 2512-2528.


Page, R. D TreeView: an application to display phylogenetic trees on personal computers.
Comput. Appl. Biosci 1996. 12: 357-358.


Pennycook, S. R and Samuels, G. J Botryosphaeria and Fusicoccum species associated with ripe fruit rot of Actinidia deliciosa (kiwifruit) in New Zealand. Mycotaxon 1985. 24: 445-458.
Phillips, A J L Botryosphaeria species associated with diseases of grapevines in Portugal. Phytopathol. Mediterr 2002. 41: 3-18.
Sakalidis, M. L, Slippers, B, Wingfield, B. D, Hardy, G. E. J and Burgess, T. I The challenge of understanding the origin, pathways and extent of fungal invasions: global populations of the
Neofusicoccum parvum-N. ribis species complex.
Divers. Distrib 2013. 19: 873-883.

Slippers, B, Crous, P. W, Denman, S, Coutinho, T. A, Wingfield, B. D and Wingfield, M. J Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as
Botryosphaeria dothidea.
Mycologia 2004. 96: 83-101.


Slippers, B, Johnson, G. I, Crous, P. W, Coutinho, T. A, Wingfield, B. D and Wingfield, M. J Phylogenetic and morphological re-evaluation of the
Botryosphaeria species causing diseases of
Mangifera indica.
Mycologia 2005. 97: 99-110.


van Niekerk, J. M, Crous, P. W, Groenewald, J. Z, Fourie, P. H and Halleen, F DNA phylogeny, morphology and pathogenicity of
Botryosphaeria species on grapevines.
Mycologia 2004. 96: 781-798.


White, T. J, Bruns, T, Lee, S and Taylor, J eds. by M. A Innis, D. H Gelfand, J. J Sninsky and T. J White, Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: A Guide to Methods and Applications, 1990. pp. 315-322. San Diego, CA, USA. Academic Press,
Zhang, L, Li, J, Hogan, S, Chung, H, Welbaum, G. E and Zhou, K Inhibitory effect of raspberries on starch digestive enzyme and their antioxidant properties and phenolic composition.
Food Chem 2010. 119: 592-599.




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print